Apologies and Warnings: users who used versions prior to 0.2.0, may need to rerun the analysis using later versions due to incorrect sequence description line assignment (in the final results, the sequence should be correctly clustered, however, sequence ID, sequence descripition in the ">" line of fasta may be incorrect.) Sincere apologies.
OrthoSLC is a pipline that performs Reciprocal Best Blast Hit (RBBH) Single Linkage Clustering to obtain Orthologous Genes, and generate core and accessory genes as final output.
It is:
- lightweight, fast, and convenient to install
- independent of relational database management systems (e.g., MySQL)
- able to handle more than 1000 genomes within hours.
The pipeline start with annotated genomes, and can produce clusters of gene id, and FASTA files of each cluster.
Note that, pipeline is recommended for sub-species level single copy core genome construction since RBBH may not work well for missions like Human-Microbe core genome construction.
Download:
$ git clone https://github.com/JJChenCharly/OrthoSLC
$ cd OrthoSLCCaveat:
The pipeline is currently available for linux-like system only and have been tested on Ubuntu 20.04 and Debian Strech.
Requirement:
- Python3 (suggest newest stable release or higher),
- C++17 and GCC/G++ 9.0 ("must" or higher for compiling) users may also directly use pre-compiled binary files by allowing excute access:
$ cd OrthoSLC
$ chmod a+x bins/*Or use install.sh to manually compile like following:
$ cd OrthoSLC
$ chmod a+x install.sh
$ ./install.sh src/ bins/- NCBI Blast+ (suggest 2.12 or higher)
Besides callable binary files, we also provide an "all-in-one" Jupyter notebook interface OrthoSLC_Python_jupyter_interface.ipynb. It is relatively slow but fits small scale analysis and allow users to do customized analysis and modification in between pipeline steps. However, for datasets with 500 or more genomes, we still recommend using the binary files, which is mainly written in C++, for optimal performance.
The programs uses A simple C++ Thread Pool implementation, and sincere thanks to its contributors.
Note:
For all steps, users do not need to make the output directory manually, program will do that for you.
Bug report:
Workflow:
For convience, it is suggested to view commandline_template.sh, before running the whole pipeline.
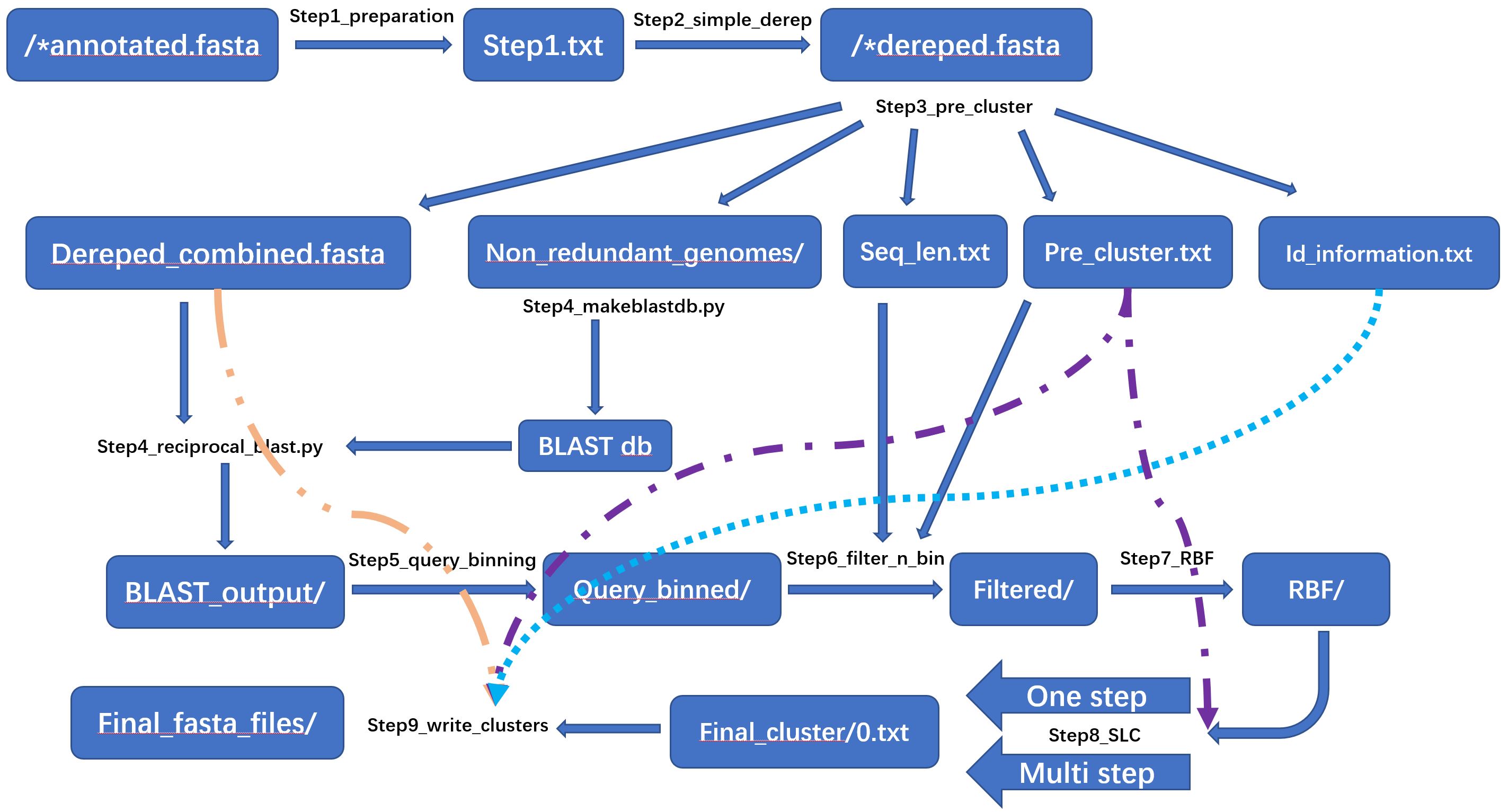
The pipeline starts with annotated genomes in fasta format. FASTA file name require strain name and extension (e.g., strain_A.ffn, strain_B.fna, strain_C.fasta etc.).
Step 1 needs the path to directory of annotated FASTA files as input, to genereate a header less, tab separated table, in which the
- first column is a short ID,
- second column is the strain name,
- third column as the absolute path.
Users can run callable binary file Step1_preparation and specifying parameters like following:
Usage: Step1_preparation -i input/ -o output.txt
-i or --input_path ---> <dir> path/to/input/directory
-o or --output_path --> <txt> path/to/output_file
-h or --help ---------> display this informationThe short ID of each genome is generated to save computational resources and storage space. Since reciprocal BLAST generates a large volume of files (millions to billions of rows if large number of genomes participated), each row contains the names of the query and subject. If the user provides input FASTA file names like:
GCA_900627445.1_PFR31F05_genomic.fastaGCA_021980615.1_PDT001237823.1_genomic.fasta
and such file names become part of gene identifier instead of the short ID used in this program, additional 30 ~ 60 GB of storage will be consumed for intermediate files and even more pressure on computing memory for analysis of 1000~ genomes.
Step 2 is to remove potential sequence duplication (e.g., copies of tRNA, some cds). This dereplication is equivalent to 100% clustering, to obtain single copy.
Step 2 requires the tab separated table output by Step 1 as input, and specifying a directory for dereplicated files.
You may run Step2_simple_derep like this:
Usage: Step2_simple_derep -i input_file -o output/ [options...]
-i or --input_path -----> <txt> path/to/file/output/by/Step1
-o or --output_path ----> <dir> path/to/output/directory
-u or --thread_number --> <int> thread number, default: 1
-h or --help -----------> display this informationAfter dereplication, users should give a careful check of size of dereplicated FASTA files. It is worth noting that if a FASTA file with a very low amount of sequences, getting processed together with all the rest, the final "core" clusters will be heavily affected and may bias your analysis.
grep -c ">" Step2_ouput_dir/* | sort -t: -k2Since the core genome construction is similar with intersection construction. It is recommend to remove some very small dereplicated fasta files BEFORE NEXT STEP, e.g., remove all dereplicated E.coli genomes with file size lower than 2.5MB as most should be larger than 4 MB.
Step 3 is the new feature since version 0.1 onward comparing with 0.1Beta.It performs 100% clustering on all dereplicated FASTAs made in Step 2.
The program of Step 3 will take the dereplicated fasta files made in step 2 as input, and produce:
- dereplicated concatenated FASTA prepared for reciprocal blast
- length of each non-redundant sequence
- pre-clustered gene id
- each genome that is redundancy-removed
- gene id information: a tab delimited table, first column as short id generated by software, second colums as description line of original fasta
You may run Step3_pre_cluster like following:
Usage: Step3_pre_cluster -i concatenated.fasta -d dereped/ -n nr_genome/ -l seq_len.txt -p pre_cluster.txt [options...]
-i or --input_path -----> <dir> path/to/directory of dereplicated FASTA from Step2
-d or --derep_fasta ----> <fasta> path/to/output/dereplicated concatenated FASTA
-n or --nr_genomes -----> <dir> path/to/directory/of/output/non-reundant/genomes
-l or --seq_len_info ---> <txt> path/to/output/sequence_length_table
-m or --id_info --------> <txt> path/to/output/id_info_table
-p or --pre_cluster ----> <txt> path/to/output/pre_clustered_file
-u or --thread_number --> <int> thread number, default: 1
-h or --help -----------> display this informationIn previous versions, program would BLAST the concatenated FASTA against each dereplicated genome sequentially, as direct all-vs-all BLAST using concatenated FASTA would be too memory intensive to run (1~ G FASTA direct all-vs-all BLAST cost roughly 120 GB memory using -mt_mode 1 with 36 threads).
As tested, BLAST the concatenated FASTA against each genome (-mt_mode 1 and 36 threads) could also be very time consuming:
- 500 Listeria monocytogenes costs > 7 hours.
- 1150 E. coli costs > 2.7 days.
However, when running the program for phylogneticlly close genmoes, there would be a high duplication level in the concatenated FASTA.
As tested, the size of concatenated FASTA could be significantly reduced after dereplication:
- 500 Listeria monocytogenes before dereplication -> ~1.33GB, after -> ~187MB,
- 1150 E. coli before dereplication -> ~5.2GB, after -> ~986MB
Besides dereplication on concatenated FASTA, this version would also use this dereplicated concatenaion to re-generate each genome without overall redundancy (non-redundant genome). Which would significantly reduce task labor.
As tested, the BLAST time usage after dereplication could be significantly reduced:
- 500 Listeria monocytogenes before dereplication -> ~7 hours, after -> ~22 mins
- 1150 E. coli before dereplication -> ~2.7 days, after -> less than 3.5 hours
Step 4 will carry out the Reciprocal Blast using NCBI Blast. You can get it from NCBI official
The pipeline will assist you to:
- Create databases for each of all dereplicated genomes using
makeblastdb. - Using
blastnorblastpto align thedereplicated concatenated FASTAagainst each of the database just made and get tabular output.
In case you have installed your blast but not exported to $PATH, you need to provide path to your blast binary file. You may use whereis blastn or whereis makeblastdb to get the full path to your blast binary file.
To create database to BLAST, users should provide path to directory where all non-redundant FASTA made in Step 4 is, and a path to output directory where BLAST database is to store.
You may run Step4_makeblastdb.py like following:
Usage: python Step4_makeblastdb.py -i input/ -o output/ [options...]
options:
-i or --input_path -----------> <dir> path/to/input/directory of nr_genomes from Step 2
-o or --output_path ----------> <dir> path/to/output/directory
-c or --path_to_makeblastdb --> <cmd_path> path/to/makeblastdb, default: makeblastdb
-u or --thread_number --------> <int> thread number, default: 1
-t or --dbtype ---------------> <str> -dbtype <String, 'nucl', 'prot'>, default: nucl
-h or --help -----------------> display this informationTo perform reciprocal BLAST, users should provide path to dereplicated concatenated FASTA producd by step 4, path to directory where databases made by makeblastdb, and a path to output directory where BLAST tabular output is to store.
You can run Step4_reciprocal_blast.py like following:
Usage: python Step4_reciprocal_blast.py -i query.fasta -o output/ -d directory_of_dbs/ [options...]
options:
-i or --query -------------> <fasta> path/to/dereped_cated.fasta from Step 3
-d or --dir_to_dbs --------> <dir> path/to/directory/of/dbs by makeblastdb
-o or --output_path -------> <dir> path/to/output/directory
-c or --path_to_blast -----> <cmd_path> path/to/blastn or blastp, default: 'blastn'
-e or --e_value -----------> <float> blast E value, default: 1e-5
-u or --blast_thread_num --> <int> blast thread number, default: 1
-m or --mem_eff_mode ------> <on/off> using memory efficient mode or not, select from <'on' or 'off'>, default: off
-f or --outfmt ------------> <str> specify blast output format if needed, unspecified means `'6 qseqid sseqid score'` as default
-t or --blastp_task ------> <str> specify blastp_task, select from <'blastp' 'blastp-fast' 'blastp-short'>, unspecified means `'blastp'` as default
-T or --blastn_task ------> <str> specify blastp_task, select from <'blastn' 'blastn-short' 'dc-megablast' 'megablast' 'rmblastn'>, unspecified means `'megablast'` as default
-h or --help --------------> display this informationThe reason to BLAST against each database sequentially rather than directly using an all-vs-all approach is to
reduce computational overhead. This can be very useful if the task involves many genomes. For example, if you have 1000 dereplicated genomes to analyze, the total size of concatenated FASTA may reach 5-10 GB. A multi-threaded BLAST job using the -mt_mode 1 by all-vs-all style could be too memory-intensive to run for such a large dataset.
In addition, sequentially running BLAST will produce one tabular output per database. This will be a better adaptation for the job parallelization of finding reciprocal best hits in later steps, which will apply the hash binning method.
Since version 0.1, program allow users to choose recoprocal BLAST executed under memory efficient mode or not. Under memory efficient mode, the real-time memory usage will be much lower but much more time consuming.
Since version 0.2.1 onward, users are allowed to set customized blast output format for other analysis requirement. If a follow up OthoSLC analysis is needed, leave the parameter as unspecified as default - "6 qseqid sseqid score" so that follow up command could parse the blast output.
This is the new feature in since version 0.1. This step is to apply hash binning to bin all presence of a query into same file to facilitate next step filtering.
You can run Step5_query_binning like following:
Usage: Step5_query_binning -i input/ -o output/ [options...]
-i or --input_path -----> <dir> path/to/input/directory of blast outputs
-o or --output_path ----> <dir> path/to/output/directory
-u or --thread_number --> <int> thread number, default: 1
-L or --bin_level ------> <int> binning level, an intger 0 < L <= 9999, default: 10
-k or --no_lock_mode ---> <on/off> select to turn no lock mode <on> or <off>, default: off
-h or --help -----------> display this informationSet bin level:
According to the amount of genomes to analysze, user should provide binning level, which is to set how many bins should be used. Level
Suggestion is that do not set the bin level too high, especially when less than 200 genomes participated. If such amount of genomes participated analysis, bin level from 10 to 100 should work as most efficient way.
As tested, an analysis of 30 genomes, has 30 BLAST output after step 4.
- A bin level of 10, takes 7 seconds to finish,
- a bin level of 100, takes 10 seconds to finish,
- a bin level of 1000, takes 24 seconds to finish,
When to set a high bin level:
Simply speaking, when you have really larger amount of genomes and not enough memory (e.g., more than 1000 genomes and less than 100 GB memory)
For example, if the output of BLAST for 1000 genomes reach 150 GB in size, and if the bin level is set to 10, there will be 10 bins to evenly distribute the data. On average, each bin will contain 1.5 GB of data, which may be too memory-intensive to process in step 5 (where requires approximately 1.5 GB of memory per bin). However, if the number of bins is increased to 1000, the size of each bin will be reduced to between 100-200 MB, it will then facilitate step 6 parallelization.
No lock mode:
we provide no lock mode in all steps that apply hash binning to speed up the process. We allow users to turn off mutex lock which is to safely write into files when multi-threading. In ours tests, program can generate files without data corruption when multi-threading with no lock (data corruption were rarely observed, the possiblity of data corruption may vary between computation platform).
This step is to filter the blast output and to apply hash binning, in order to provide best preparation for reciprocal best find.
Step 6 requires path to directory of query binning output (Step 5), sequence length information, pre-cluster information output by Step 3 as input.
You can run Step6_filter_n_bin like following:
Usage: Step6_filter_n_bin -i input/ -o output/ -s seq_len_info.txt [options...]
-i or --input_path --------> <dir> path/to/input/directory from Step 5
-o or --output_path -------> <dir> path/to/output/directory
-s or --seq_len_path ------> <txt> path/to/seq_len_info.txt from Step 3
-p or --pre_cluster_path --> <txt> path/to/pre_cluster.txt from Step 3
-L or --bin_level ---------> <int> binning level, an intger 0 < L <= 9999 , default: 10
-r or --length_limit ------> <float> length difference limit, 0 < r <= 1, default: 0.3
-k or --no_lock_mode ------> <on/off> select to turn no lock mode <on> or <off>, default: off
-u or --thread_number -----> <int> thread number, default: 1
-h or --help --------------> display this informationThe pipeline will carry out following treatment to BLAST output:
- Paralog removal:
If query and subject is from same strain, the hit will be skipped, as to remove paralog. - Length ratio filtering:
Within a hit, query length$Q$ and subject length$S$ , the ratio$v$ of this 2 length
should be within a range, according to L. Salichos et al,
If above condition not met, the hit will be removed from analysis.
- Non-best-hit removal:
- Identical sequences are always regarded as best hit.
- If a query has more than 1 subject hits, only the query-subject pair with highest score will then be kept.
- if pairs are of same score, the pair whose query and subject are of more similar length will be kept.
- Sorting and binning:
For every kept hit, its query and subject will be sorted using Python or C++ built in sort algorithm. This is because in a sequential blast output file, only "single direction best hit" can be obtained, its "reciprocal best hit" only exist in other files, which poses difficulty doing "repriprocal finding".
However, if a query$a$ and its best suject hit$b$ , passed filter above, and form$(a, b)$ , and in the mean time we sort its rericprocal hit$(b, a)$ from another file into$(a, b)$ , then both$(a, b)$ will generate same hash value. This hashed value with last several digits will allow us to bin them into same new file. Therefore, after this binning, "reciprocal finding" will be turned into "duplication finding" within one same file.
Set bin level:
According to the amount of genomes to analysze, user should provide binning level, which is to set how many bins should be used. Level
Suggestion is that do not set the bin level too high, especially when less than 200 genomes participated. If such amount of genomes participated analysis, bin level from 10 to 100 should work as most efficient way.
When to set a high bin level:
Simply speaking, when you have really larger amount of genomes and not enough memory (e.g., more than 1000 genomes and less than 100 GB memory)
for example, if the output of BLAST for 1000 genomes reach 200 GB in size, and if the bin level is set to 100, there will be 100 bins to evenly distribute the data. On average, each bin will contain 1.7 GB of data, which may be too memory-intensive to process in step 7, where reciprocal find is performed (which requires approximately 1.7 GB of memory per bin). However, if the number of bins is increased to 1000, the size of each bin will be reduced to between 100-200 MB, which then facilitate step 7 parallelization.
Note:
This is the one of the most computation and I/O intensive step, use the C++ based binary file to process for better efficiency.
This Step is to find reciprocal best hits. In Step 6, query-subject pairs had been binned into different files according to their hash value, therefore, pair
In addition, Step 7 also does hash binning after a reciprocal best hit is comfirmed. Query-subject pairs will be binned by the hash value of query ID, which then put pairs with common elements into same bin to assist faster clustering in next step.
Step 7 requires path to directory of bins output by Step 6, and path to output directory.
You can run Step7_RBF like following:
Usage: Step7_RBF -i input/ -o output/ [options...]
-i or --input_path -----> <dir> path/to/input/directory from Step 6
-o or --output_path ----> <dir> path/to/output/directory
-u or --thread_number --> <int> thread number, default: 1
-k or --no_lock_mode ---> <on/off> select to turn no lock mode <on> or <off>, default: off
-L or --bin_level ------> <int> binning level, an intger 0 < L <= 9999 , default: 10
-h or --help -----------> display this informationSet bin level:
According to the amount of genomes to analysze, user should provide binning level, which is to set how many bins should be used. Level
Suggestion is that do not set the bin level too high, especially when less than 200 genomes participated. If such amount of genomes participated analysis, bin level from 10 to 100 should work as most efficient way.
As tested, 30 genomes, if 10 bins generated by Step 7:
- A bin level of 10, takes 1 seconds to finish,
- a bin level of 100, takes 2 seconds to finish,
- a bin level of 1000, takes 7 seconds to finish,
When to set a high bin level:
Simply speaking, when you have really larger amount of genomes and not enough memory (e.g., more than 1000 genomes and less than 100 GB memory)
Less bins could make step 7 faster, but step 8 more memory intensive.
Note:
This is the one of the most computation and I/O intensive step, use the C++ based binary file to process for better efficiency.
This step will carry out single linkage clustering on output from step 7. Users may perform "multi-step-to-final" or "one-step-to-final" clustering by adjusting the compression_size parameter. In the output files, each row is a cluster (stopped by "\n") and each gene ID is separated by "\t".
In case that large amount genomes participated analysis, it could be memory intensive to reach final cluster in a single step. The pipeline provide ability to extenuate such pressure by reaching final cluster with multiple steps. For example, if compression_size = 5 is provided, program will perform clustering using 5 files at a time and shrink the output file number by a factor of 5.
Note Before Start
User must specify the path to pre-cluster file produced in Step 3, when running the LAST step of multi step to final, or when running direct one step to final.
You can run Step8_SLC like following:
Usage: Step8_SLC -i input/ -o output/ [options...]
options:
-i or --input_path --------> <dir> path/to/input/directory from Step 7
-o or --output_path -------> <dir> path/to/output/directory
-u or --thread_number -----> <int> thread number, default: 1
-p or --pre_cluster_path --> <txt> path/to/output/pre_clustered file from Step 3
-S or --compression_size --> <int> compression size, default: 10, 'all' means one-step-to-final
-h or --help --------------> display this informationExample running with multi-step-to-final approach:
E.g., there are 1,000 files generated by Step 8
Set -S or --compression_size to 1
bin_dir="bin_dirctory"
wd="working_dir"
time $bin_dir/Step8_SLC \
-i $wd"/S8_op" \
-o $wd"/SLC_1" \
-u 16 \
-S 1above commands will perform clustering on every 1 file, and generate 1,000 files in $cp"/SLC_1".
Set -S or --compression_size to 5
time $bin_dir/Step8_SLC \
-i $wd"/SLC_1" \
-o $wd"/SLC_2" \
-u 16 \
-S 5it will perform clustering on every 5 files, and generate 200 files in $cp"/SLC_2".
Set -S or --compression_size to 10
time $bin_dir/Step8_SLC \
-i $cp"/SLC_2" \
-o $cp"/SLC_3" \
-u 16 \
-S 10it will perform clustering on every 10 files, and generate 20 files in $cp"/SLC_3.
Finally, Set -S or --compression_size as all, and provide pre_cluster.txt made in Step 4 using -p or --pre_cluster_path
time $bin_dir/Step8_SLC \
-i $cp"/SLC_3" \
-o $cp"/SLC_final" \
-p $cp"/S3_op_pre_cluster.txt" \
-u 16 \
-S allit will perform clustering on on all, and give the final 1 cluster file in $cp"/SLC_final.
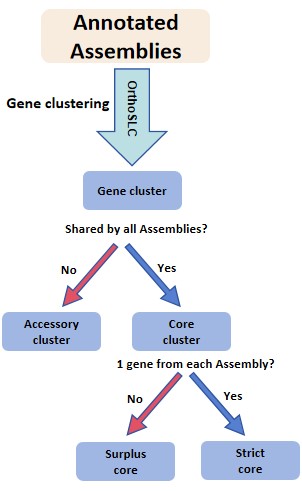
In Step 9, program will help user to generate FASTA file for each cluster. By providing the final one cluster file generated by Step 8 as input, program produces 3 types of clusters into 3 directories separately.
Noteably, those genomes
- depreplicated in Step 2,
- not removed because of too low genome size
- participated processes up to this step,
ls S2_op_dir/ | cat -n | tailare used to separate 3 types of clusters.
- In drectory
accessory_cluster(a cluster not shared by all genomes), FASTA files of clusters which do not have genes from all genomes participated analysis, will be output in this drectory. For example, there are 100 genomes in analysis, a cluster with less than 100 genes will have its FASTA output here. Also, if a cluster has >= 100 genes, but all these genes are from less than 100 genomes, its FASTA will be in this directory. - In drectory
strict_core, a give cluster with exactly 1 gene from every genome analyzed. Such clusters will have their FASTA files here. - In drectory
surplus_core, a give cluster with at least 1 gene from every genome analyzed, and some genomes has more than 1 genes in this cluster. Such clusters will have their FASTA files here.
This step also requires the concatenated FASTA made in Step 3 as input.
You may run Step9_write_clusters like this:
Usage: Step9_write_clusters -i input_path -o output/ -f concatenated.fasta [options...]
options:
-i or --input_path --------> <dir> path/to/input/final_cluster_file from Step 8
-o or --output_path -------> <dir> path/to/output/directory
-f or --fasta_path --------> <fasta> path/to/dereped_cated_fasta from Step 3
-m or --id_info_path ------> <txt> path/to/output/id_info_table from Step 3
-p or --pre_cluster_path --> <txt> path/to/pre_clustered_file from Step 3
-c or --total_count -------> <int> amonut of genomes to analyze
-t or --cluster_type ------> <txt> select from < accessory / strict / surplus >, separate by ',', all types if not specified
-u or --thread_number -----> <int> thread number, default: 1
-h or --help --------------> display this information