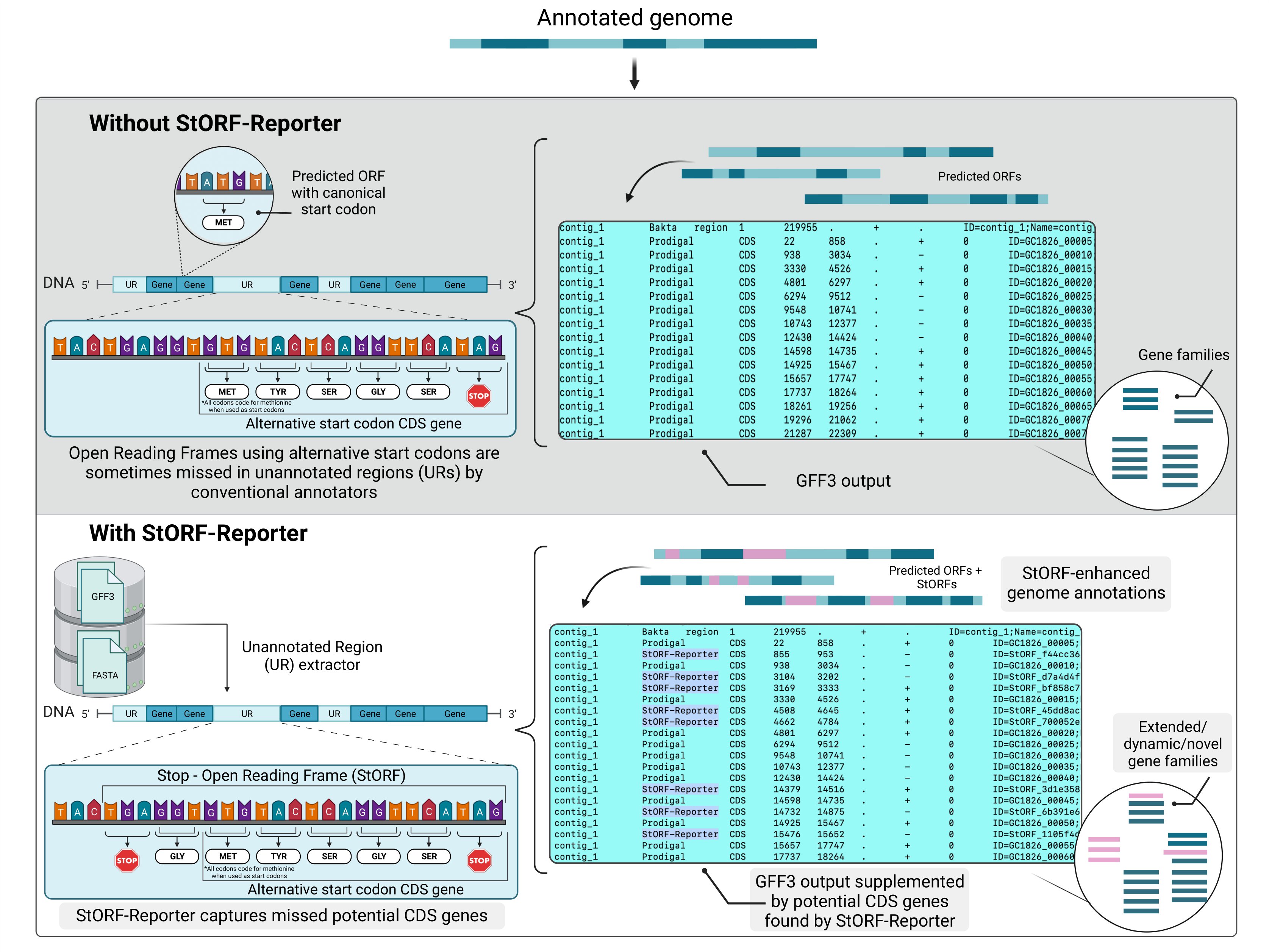
StORF-Reporter has now been published in NAR: https://doi.org/10.1093/nar/gkad814
StORF-Reporter, a toolkit that returns missed CDS genes from the Unannotated Regions (URs) of prokaryotic genomes.
This will also install the python-standard library numpy (>=1.22.0,<1.24.0), Pyrodigal - (https://github.com/althonos/pyrodigal) and ORForise (https://github.com/NickJD/ORForise).
Consider using '--no-cache-dir' with pip to ensure the download of the newest version of StORF-Reporter.
Please Note: To report Con-StORFs (Pseudogenes and genes that have alternative use of stop codons), use "-con_storfs True". To disable the reporting of StORFs use "-con_only".
#############################################################
Supplement a current annotation from a tool such as Prokka or Bakta. A new GFF file will be created compatible with downstream pangenome analysis tools such as Roary and Panaroo.
For use on a single Prokka/Bakta output directory - Will also create a new fasta file with Prokka/Bakta genes and StORF sequences.
StORF-Reporter -anno Prokka Out_Dir -p .../Test_Datasets/Prokka_E-coli/For use on multiple Prokka/Bakta output directies - Will also create a new fasta file with Prokka/Bakta genes and StORF sequences.
StORF-Reporter -anno Prokka Multiple_Out_Dirs -p ../Test_Datasets/Multi_Prokka_OutsStORF-Reporter -anno Prokka Multiple_GFFs -p .../Test_Datasets/Prokka_Outputs/For use on a GFF file from a CDS prediction tool such as Prodigal - Provide a GFF file and StORF-Reporter will find the matching .fa/.fasta/.fna (must have the same name).
StORF-Reporter -anno Feature_Types Single_Genome -p .../Test_Datasets/Matching_GFF_FASTA/Myco.gffFor use on a directory containing multiple GFF files from a CDS prediction tool such as Prodigal - StORF-Reporter will find the matching .fa/.fasta/.fna (must have the same name).
StORF-Reporter -anno Feature_Types Multiple_Genomes -p .../Test_Datasets/Matching_GFF_FASTA/StORF-Reporter -anno Feature_Types Multiple_Combined_GFFs -p .../Test_Datasets/Combined_GFFs/To perform a fresh end-to-end annotation of a genome without an annotation, StORF-Reporter will use Pyrodigal to predict CDS genes and then supplement with StORFs.
StORF-Reporter -anno Pyrodigal Single_FASTA -p .../Test_Datasets/Pyrodigal/E-coli.faStORF-Reporter -anno Ensembl Single_Genome -p .../Test_Datasets/Matching_GFF_FASTA/E-coli.gffusage: StORF_Reporter.py [-h]
[-anno [{Prokka,Bakta,Out_Dir,Multiple_Out_Dirs,Single_GFF,Multiple_GFFs,Ensembl,Feature_Types,Single_Genome,Multiple_Genomes,Single_Combined_GFF,Multiple_Combined_GFFs,Pyrodigal,Single_FASTA,Multiple_FASTA} ...]]
[-p PATH] [-af ALT_FILENAME] [-oname O_NAME] [-odir O_DIR] [-sout {True,False}] [-lw {True,False}] [-aa {True,False}] [-gz {True,False}] [-py_train [{longest,individual,meta}]] [-py_fasta {True,False}]
[-py_unstorfed {True,False}] [-gene_ident GENE_IDENT] [-min_len MINLEN] [-max_len MAXLEN] [-ex_len EXLEN] [-spos {True,False}] [-rs {True,False}] [-con_storfs {True,False}] [-con_only {True,False}]
[-ps {True,False}] [-wc {True,False}] [-short_storfs {False,Nolap,Olap}] [-short_storfs_only {True,False}] [-minorf MIN_ORF] [-maxorf MAX_ORF] [-codons STOP_CODONS]
[-olap_filt [{none,single-strand,both-strand}]] [-start_filt {True,False}] [-so [{start_pos,strand}]] [-f_type [{StORF,CDS,ORF}]] [-olap OVERLAP_NT] [-ao ALLOWED_OVERLAP] [-overwrite {True,False}]
[-verbose {True,False}] [-v]
StORF-Reporter v1.3.4: StORF-Reporter Run Parameters.
Required Options:
-anno [{Prokka,Bakta,Out_Dir,Multiple_Out_Dirs,Single_GFF,Multiple_GFFs,Ensembl,Feature_Types,Single_Genome,Multiple_Genomes,Single_Combined_GFF,Multiple_Combined_GFFs,Pyrodigal,Single_FASTA,Multiple_FASTA} ...]
Select Annotation and Input options for one of the 3 options listed below
### Prokka/Bakta Annotation Option 1:
Prokka = Report StORFs for a Prokka annotation;
Bakta = Report StORFs for a Bakta annotation;
--- Prokka/Bakta Input Options:
Out_Dir = To provide the output directory of either a Prokka or Bakta run (will produce a new GFF and FASTA file containing original and extended annotations);
Multiple_Out_Dirs = To provide a directory containing multiple Prokka/Bakta standard output directories - Will run on each sequentially;
Single_GFF = To provide a single Prokka or Bakta GFF - searches for accompanying ".fna" file (will provide a new extended GFF);
Multiple_GFFs = To provide a directory containing multiple Prokka or Bakta GFF files - searches for accompanying ".fna" files (will provide a new extended GFF);
### Standard GFF Annotation Option 2:
Ensembl = Report StORFs for an Ensembl Bacteria annotation (ID=gene);
Feature_Types = Used in conjunction with -gene_ident to define features such as CDS,rRNA,tRNA for UR extraction (default CDS);
--- Standard GFF Input Options:
Single_Genome = To provide a single Genome - accompanying FASTA must share same name as given gff file (can be .fna, .fa or .fasta);
Multiple_Genomes = To provide a directory containing multiple accompanying GFF and FASTA files - files must share the same name (fasta can be .fna, .fa or .fasta);
Single_Combined_GFF = To provide a GFF file with embedded FASTA at the bottom;
Multiple_Combined_GFFs = To provide a directory containing multiple GFF files with embedded FASTA at the bottom;
### Complete Annotation Option 3:
Pyrodigal = Run Pyrodigal then Report StORFs (provide path to single FASTA or directory of multiple FASTA files ;
--- Complete Annotation Input Options:
Single_FASTA = To provide a single FASTA file;
Multiple_FASTA = To provide a directory containing multiple FASTA files (will detect .fna,.fa,.fasta);
-p PATH Provide input file or directory path
StORF-Reporter Options:
-af ALT_FILENAME Default - Prokka/Bakta output directory share the same prefix with their gff/fna files - Use this option when Prokka/Bakta output directory name is different from the gff/fna files within and StORF-Reporter
will search for the gff/fna with the given prefix (MyProkkaDir/"altname".gff) - Does not work with "Multiple_Out_Dirs" option
-oname O_NAME Default - Appends '_StORF-Reporter_Extended' to end of input filename - Takes the directory name of Prokka/Bakta output if given as input or the input for -af if given - Multiple_* runs will be numbered
-odir O_DIR Default - Same directory as input
-sout {True,False} Default - False: Print out StORF sequences separately from Prokka/Bakta annotations
-lw {True,False} Default - True: Line wrap FASTA sequence output at 60 chars
-aa {True,False} Default - False: Report StORFs as amino acid sequences
-gz {True,False} Default - False: Output as .gz
Pyrodigal Options:
-py_train [{longest,individual,meta}]
Default - longest: Type of model training to be done for Pyrodigal CDS prediction: Options: longest = Trains on longest contig; individual = Trains on each contig separately - runs in meta mode if contig is
< 20KB; meta = Runs in meta mode for all sequences
-py_fasta {True,False}
Default - False: Output Pyrodigal+StORF predictions in FASTA format
-py_unstorfed {True,False}
Default - False: Provide GFF containing original Pyrodigal predictions
UR-Extractor Options:
-gene_ident GENE_IDENT
Identifier used for extraction of Unannotated Regions such as "misc_RNA,gene,mRNA,CDS,rRNA,tRNA,tmRNA,CRISPR,ncRNA,regulatory_region,oriC,pseudo" - To be used with "-anno Feature_Types" - "-gene_ident
Prokka" will select features present in Prokka annotations
-min_len MINLEN Default - 30: Minimum UR Length
-max_len MAXLEN Default - 100,000: Maximum UR Length
-ex_len EXLEN Default - 50: UR Extension Length
StORF-Finder Options:
-spos {True,False} Default - False: Output StORF positions inclusive of first stop codon
-rs {True,False} Default - True: Remove stop "*" from StORF amino acid sequences
-con_storfs {True,False}
Default - False: Output Consecutive StORFs
-con_only {True,False}
Default - False: Only output Consecutive StORFs
-ps {True,False} Default - False: Partial StORFs reported
-wc {True,False} Default - False: StORFs reported across entire sequence
-short_storfs {False,Nolap,Olap}
Default - False: Run StORF-Finder in "Short-StORF" mode. Will only return StORFs between 30 and 120 nt that do not overlap longer StORFs - Only works with StORFs for now. "Nolap" will filter Short-StORFs
which areoverlapped by StORFs and Olap will report Short-StORFs which do overlap StORFs. Overlap is defined by "-olap".
-short_storfs_only {True,False}
Default - True. Only report Short-StORFs?
-minorf MIN_ORF Default - 99: Minimum StORF size in nt
-maxorf MAX_ORF Default - 60kb: Maximum StORF size in nt
-codons STOP_CODONS Default - ('TAG,TGA,TAA'): List Stop Codons to use
-olap_filt [{none,single-strand,both-strand}]
Default - "both-strand": Filtering level "none" is not recommended, "single-strand" for single strand filtering and both-strand for both-strand longest-first tiling
-start_filt {True,False}
Default - False: Filter out StORFs without at least one of the 3 common start codons (best used for short-storfs).
-so [{start_pos,strand}]
Default - Start Position: How should StORFs be ordered when >1 reported in a single UR.
-f_type [{StORF,CDS,ORF}]
Default - "CDS": Which GFF feature type for StORFs to be reported as in GFF - "CDS" is probably needed for use in tools such as Roary and Panaroo
-olap OVERLAP_NT Default - 50: Maximum number of nt of a StORF which can overlap another StORF.
-ao ALLOWED_OVERLAP Default - 50 nt: Maximum overlap between a StORF and an original gene.
Misc:
-overwrite {True,False}
Default - False: Overwrite StORF-Reporter output if already present
-verbose {True,False}
Default - False: Print out runtime messages
-v Print out version number and exit###################################
UR-Extractor -f .../Test_Datasets/Matching_GFF_FASTA/E-coli.fa -gff .../Test_Datasets/Matching_GFF_FASTA/E-coli.gffusage: StORF_Extractor.py [-h] [-storf_input {Combined,Separate}] [-p PATH] [-gff_out {True,False}] [-aa {True,False}]
[-lw {True,False}] [-stop_ident {True,False}] [-oname O_NAME] [-odir O_DIR] [-gz {True,False}]
[-verbose {True,False}] [-v]
Single_Genome v1.3.4: StORF-Extractor Run Parameters.
Required Arguments:
-storf_input {Combined,Separate}
Are StORFs to be extracted from Combined GFF/FASTA or Separate GFF/FASTA files?
-p PATH Provide input files or directory path
Output:
-gff_out {True,False}
Default - False: Output StORFs in GFF format
-aa {True,False} Default - False: Report StORFs as amino acid sequences
-lw {True,False} Default - True: Line wrap FASTA sequence output at 60 chars
-stop_ident {True,False}
Default - True: Identify Stop Codon positions with '*'
-oname O_NAME Default - Appends '_Extracted_StORFs' to end of input GFF filename
-odir O_DIR Default - Same directory as input FASTA
-gz {True,False} Default - False: Output as .gz
Misc:
-verbose {True,False}
Default - False: Print out runtime messages
-v Default - False: Print out version number and exitStORF-Finder -f .../Test_Datasets/Matching_GFF_FASTA/E-coli_UR.fa usage: StORF_Finder.py [-h] [-f FASTA] [-ua {True,False}] [-wc {True,False}] [-ps {True,False}] [-olap_filt [{none,single-strand,both-strand}]] [-start_filt {True,False}] [-con_storfs {True,False}] [-con_only {True,False}] [-short_storfs {False,Nolap,Olap}] [-short_storfs_only {True,False}]
[-stop_ident {True,False}] [-f_type [{StORF,CDS,ORF}]] [-minorf MIN_ORF] [-maxorf MAX_ORF] [-codons STOP_CODONS] [-olap OVERLAP_NT] [-s SUFFIX] [-so [{start_pos,strand}]] [-spos {True,False}] [-oname O_NAME] [-odir O_DIR] [-gff {True,False}] [-aa {True,False}] [-aa_only {True,False}]
[-lw {True,False}] [-gff_fasta {True,False}] [-gz {True,False}] [-verbose {True,False}] [-v]
StORF-Reporter v1.3.4: StORF-Finder Run Parameters.
Required Arguments:
-f FASTA Input FASTA File - (UR_Extractor output)
Optional Arguments:
-ua {True,False} Default - Treat input as Unannotated: Use "-ua False" for standard fasta
-wc {True,False} Default - False: StORFs reported across entire sequence
-ps {True,False} Default - False: Partial StORFs reported
-olap_filt [{none,single-strand,both-strand}]
Default - "both-strand": Filtering level "none" is not recommended, "single-strand" for single strand filtering and both-strand for both-strand longest-first tiling
-start_filt {True,False}
Default - False: Filter out StORFs without at least one of the 3 common start codons (best used for short-storfs).
-con_storfs {True,False}
Default - False: Output Consecutive StORFs
-con_only {True,False}
Default - False: Only output Consecutive StORFs
-short_storfs {False,Nolap,Olap}
Default - False: Run StORF-Finder in "Short-StORF" mode. Will only return StORFs between 30 and 120 nt that do not overlap longer StORFs - Only works with StORFs for now. "Nolap" will filter Short-StORFs which areoverlapped by StORFs and Olap will report Short-StORFs which do overlap StORFs.
Overlap is defined by "-olap".
-short_storfs_only {True,False}
Default - True. Only report Short-StORFs?
-stop_ident {True,False}
Default - True: Identify Stop Codon positions with '*'
-f_type [{StORF,CDS,ORF}]
Default - "StORF": Which GFF feature type for StORFs to be reported as in GFF
-minorf MIN_ORF Default - 99: Minimum StORF size in nt
-maxorf MAX_ORF Default - 60kb: Maximum StORF size in nt
-codons STOP_CODONS Default - ('TAG,TGA,TAA'): List Stop Codons to use
-olap OVERLAP_NT Default - 50: Maximum number of nt of a StORF which can overlap another StORF.
-s SUFFIX Default - Do not append suffix to genome ID
-so [{start_pos,strand}]
Default - Start Position: How should StORFs be ordered when >1 reported in a single UR.
-spos {True,False} Default - False: Print out StORF positions inclusive of first stop codon
Output:
-oname O_NAME Default - Appends '_StORF-R' to end of input FASTA filename
-odir O_DIR Default - Same directory as input FASTA
-gff {True,False} Default - True: Output a GFF file
-aa {True,False} Default - False: Report StORFs as amino acid sequences
-aa_only {True,False}
Default - False: Only output Amino Acid Fasta
-lw {True,False} Default - True: Line wrap FASTA sequence output at 60 chars
-gff_fasta {True,False}
Default - False: Report all gene sequences (nt) at the bottom of GFF files in Prokka output mode
-gz {True,False} Default - False: Output as .gz
Misc:
-verbose {True,False}
Default - False: Print out runtime messages
-v Default - False: Print out version number and exitSubpackage to extract sequences reported by StORF-Reporter from a genome annotation.
StORF-Extractor -storf_input Combined -p .../Test_Datasets/Combined_GFFs/E-coli_Combined_StORF-Reporter_Extended.gff usage: StORF_Extractor.py [-h] [-storf_input {Combined,Separate}] [-p PATH] [-gff_out {True,False}] [-oname O_NAME] [-odir O_DIR] [-gz {True,False}] [-verbose {True,False}] [-v]
StORF-Reporter v1.3.4: StORF-Extractor Run Parameters.
Required Arguments:
-storf_input {Combined,Separate}
Are StORFs to be extracted from Combined GFF/FASTA or Separate GFF/FASTA files?
-p PATH Provide input file or directory path
Output:
-gff_out {True,False}
Default - False: Output StORFs in GFF format
-oname O_NAME Default - Appends '_Extracted_StORFs' to end of input GFF filename
-odir O_DIR Default - Same directory as input FASTA
-gz {True,False} Default - False: Output as .gz
Misc:
-verbose {True,False}
Default - False: Print out runtime messages
-v Default - False: Print out version number and exitSubpackage to remove sequences reported by StORF-Reporter without a Blast/Diamond hit (any alignment in BLAST 6 format).
StORF-Remover -gff .../Test_Datasets/StORF_Extractor_And_Remover/Myco_UR_StORF-R.gff -blast .../Test_Datasets/StORF_Extractor_And_Remover/Myco_URs_StORFs_aa_Swiss.tab usage: StORF_Remover.py [-h] [-gff GFF] [-blast BLAST] [-min_score MINSCORE] [-oname O_NAME] [-odir O_DIR] [-gz {True,False}]
[-verbose {True,False}] [-v]
StORF-Reporter v1.3.4: UR-Remover Run Parameters.
Required Arguments:
-gff GFF GFF annotation file for the FASTA
-blast BLAST BLAST format 6 annotation file
Optional Arguments:
-min_score MINSCORE Minimum BitScore to keep StORF: Default 30
Output:
-oname O_NAME Default - Appends '_UR' to end of input GFF filename
-odir O_DIR Default - Same directory as input GFF
-gz {True,False} Default - False: Output as .gz
Misc:
-verbose {True,False}
Default - False: Print out runtime messages
-v Default - False: Print out version number and exit