This is a reproducible benchmark of structural variants in challenging medically relevant genes. The truth set is described in detail here:
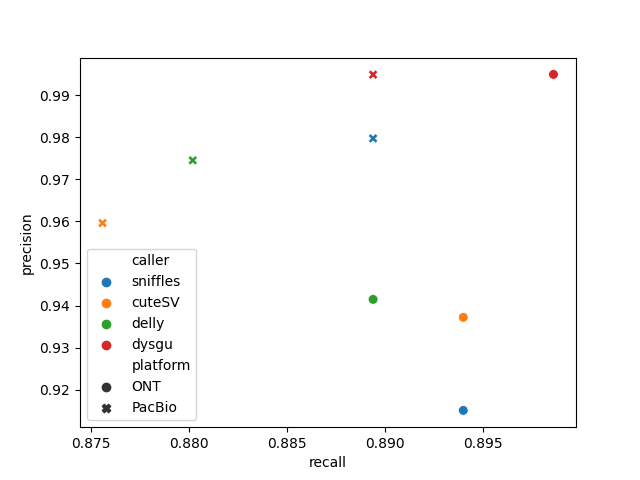
| caller | platform | TP | FP | FN | precision | recall | f1 | gt_concordance | |
|---|---|---|---|---|---|---|---|---|---|
| 0 | sniffles | ONT | 194 | 18 | 23 | 0.9151 | 0.894 | 0.9044 | 0.8814 |
| 1 | cuteSV | ONT | 194 | 13 | 23 | 0.9372 | 0.894 | 0.9151 | 0.8918 |
| 2 | delly | ONT | 193 | 12 | 24 | 0.9415 | 0.8894 | 0.9147 | 0.8964 |
| 3 | dysgu | ONT | 195 | 1 | 22 | 0.9949 | 0.8986 | 0.9443 | 0.8821 |
| caller | platform | TP | FP | FN | precision | recall | f1 | gt_concordance | |
|---|---|---|---|---|---|---|---|---|---|
| 0 | sniffles | PacBio | 193 | 4 | 24 | 0.9797 | 0.8894 | 0.9324 | 0.8912 |
| 1 | cuteSV | PacBio | 190 | 8 | 27 | 0.9596 | 0.8756 | 0.9157 | 0.9 |
| 2 | delly | PacBio | 191 | 5 | 26 | 0.9745 | 0.8802 | 0.9249 | 0.8743 |
| 3 | dysgu | PacBio | 193 | 1 | 24 | 0.9948 | 0.8894 | 0.9392 | 0.8808 |
Reads were from Oxford Nanopore kit14 (~40X coverage), and PacBio Revio HiFi (~30X coverage). SV callers tested were as follows:
For benchmarking truvari v4.0.0 was used with parameters -r 1000 --passonly
Run the benchmark.sh script or follow along below.
- ~ 200 Gb space, 32 gb Ram, 4 cores
- Docker / Singularity (Required for Mac, optional for Linux)
mkdir benchmark && cd benchmark
docker run -it --memory="32g" --mount src="${PWD}",target=/results,type=bind condaforge/mambaforge
mamba update conda -y && cd results
Note, you may need to set the memory and swap space manually using Docker Desktop on Mac.
Install tools:
mamba create -c bioconda -c conda-forge -n bench python=3.9 awscli sniffles=2.2.0 cuteSV=2.0.3 truvari=4.0.0 delly=1.1.6 -y
conda activate bench
pip install dysgu==1.6.0
Reference genome:
wget https://ftp.ncbi.nlm.nih.gov/genomes/all/GCA/000/001/405/GCA_000001405.15_GRCh38/seqs_for_alignment_pipelines.ucsc_ids/GCA_000001405.15_GRCh38_no_alt_analysis_set.fna.gz
wget https://ftp.ncbi.nlm.nih.gov/genomes/all/GCA/000/001/405/GCA_000001405.15_GRCh38/seqs_for_alignment_pipelines.ucsc_ids/GCA_000001405.15_GRCh38_no_alt_analysis_set.fna.fai
gunzip GCA_000001405.15_GRCh38_no_alt_analysis_set.fna.gz
ref=GCA_000001405.15_GRCh38_no_alt_analysis_set.fna
Oxford Nanopore reads https://labs.epi2me.io/giab-2023.05/:
aws s3 sync --no-sign-request --include='PAO89685.pass.cram*' --exclude="*fail*" s3://ont-open-data/giab_2023.05/analysis/hg002/sup/ .
PacBio reads https://www.pacb.com/connect/datasets/:
wget https://downloads.pacbcloud.com/public/revio/2022Q4/HG002-rep1/analysis/HG002.m84011_220902_175841_s1.GRCh38.bam
wget https://downloads.pacbcloud.com/public/revio/2022Q4/HG002-rep1/analysis/HG002.m84011_220902_175841_s1.GRCh38.bam.bai
SV truth set:
r=ftp://ftp-trace.ncbi.nlm.nih.gov/giab/ftp/data/AshkenazimTrio/analysis/NIST_HG002_medical_genes_SV_benchmark_v0.01/HG002_GRCh38_difficult_medical_gene_SV_benchmark_v0.01
wget ${r}.bed && wget ${r}.vcf.gz && wget ${r}.vcf.gz.tbi
ONT reads:
sniffles --input PAO89685.pass.cram --vcf HG002.PAO89685.sniffles.vcf
dysgu run --mode nanopore --procs 4 -x --clean $ref wd PAO89685.pass.cram > HG002.PAO89685.dysgu.vcf
mkdir wd_cuteSV
cuteSV -t 4 -s 3 --genotype PAO89685.pass.cram $ref HG002.PAO89685.cuteSV.vcf wd_cuteSV
delly lr -g $ref PAO89685.pass.cram > HG002.PAO89685.delly.vcf
PacBio reads:
sniffles --input HG002.m84011_220902_175841_s1.GRCh38.bam --vcf HG002.pacbio.sniffles.vcf
dysgu call --mode pacbio --procs 4 -x --clean $ref wd HG002.m84011_220902_175841_s1.GRCh38.bam > HG002.pacbio.dysgu.vcf
mkdir wd_cuteSV
cuteSV -t 4 -s 3 --genotype HG002.m84011_220902_175841_s1.GRCh38.bam $ref HG002.pacbio.cuteSV.vcf wd_cuteSV
delly lr -g $ref HG002.m84011_220902_175841_s1.GRCh38.bam > HG002.pacbio.delly.vcf
ONT data:
callers=( "sniffles" "cuteSV" "delly" "dysgu" )
for name in "${callers[@]}"
do
bgzip HG002.PAO89685.${name}.vcf
tabix HG002.PAO89685.${name}.vcf.gz
truvari bench -f GCA_000001405.15_GRCh38_no_alt_analysis_set.fna \
-b HG002_GRCh38_difficult_medical_gene_SV_benchmark_v0.01.vcf.gz \
--includebed HG002_GRCh38_difficult_medical_gene_SV_benchmark_v0.01.bed \
-c HG002.PAO89685.${name}.vcf.gz \
--passonly -r 1000 \
-o truvari_${name}
done
PacBio data:
callers=( "sniffles" "cuteSV" "delly" "dysgu" )
for name in "${callers[@]}"
do
bgzip HG002.pacbio.${name}.vcf
tabix HG002.pacbio.${name}.vcf.gz
truvari bench -f GCA_000001405.15_GRCh38_no_alt_analysis_set.fna \
-b HG002_GRCh38_difficult_medical_gene_SV_benchmark_v0.01.vcf.gz \
--includebed HG002_GRCh38_difficult_medical_gene_SV_benchmark_v0.01.bed \
-c HG002.pacbio.${name}.vcf.gz \
--passonly -r 1000 \
-o truvari_${name}
done
Run the plotting script:
python3 plot_benchmark.py