nat-tech
The goal of nat-tech is to provide R client utilities for streamlining
registration of light-level microscopy data to existing template
brains
for for D. melanogaster neurons. It is a set of R scripts that enable
a user to register raw light miscroscopy data, and create .nrrd files of
the final registered product alongside hemibrain neuron reconstructions.
Our main use case is in running a splitGAL4 line screen; we want to see
whether the potential hits in our screen co-localise with the specified
hemibrain neuronal cell types we are trying to target. This tool
utilizes the Computational Morphometry Toolkit
(CMTK) to write CMTK registration
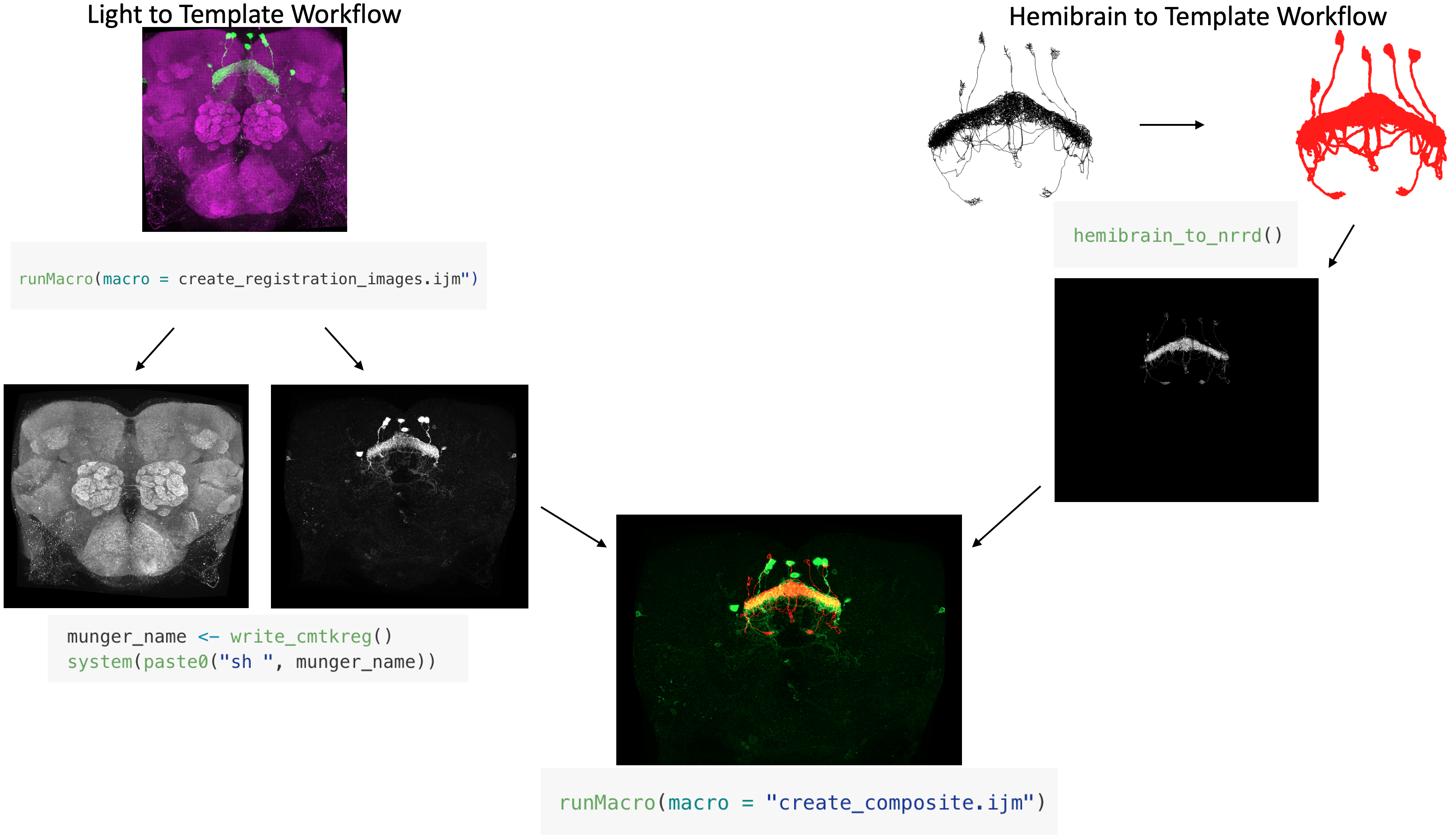
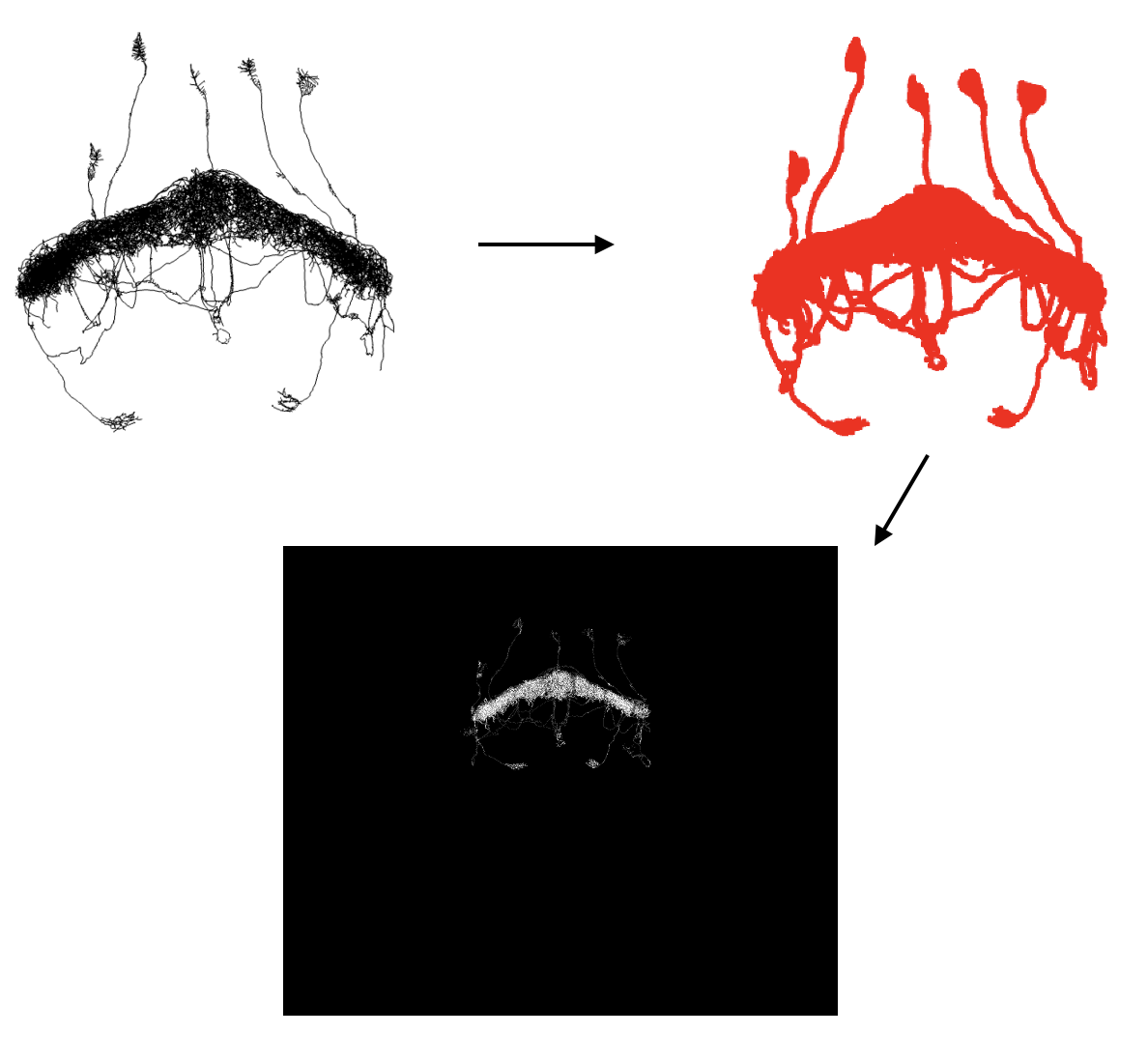
commands. Below is a graphic detailing how this tool works and the
expected output.
Data sets
The major EM dataset at the time when this package was built was the hemibrain connectome. Connectome data can be seen using the neuPrint website and accessed programmatically in R using neuprintr. This tool also will help you co-visualize the registered data to the corresponding neuron in the hemibrain connectome. The user must specifcy what cell type they want included in the hemibrain .nrrd file.
How have these registrations been performed?
In the past, light-level microscopy data could be registrated using the fiji-cmtk-gui. This tool automates this process so the user doesn’t have to open the GUI or FIJI and also adds functionality by co-plotting these neurons with connectome date.
In the following, we detail some of nat-tech’s functionality
Useful Functions
#takes a .nrrd file and plots it with its counterpart in hemibrain connectome
nrrd_to_hemibrain()
#converts a .swc neuron trace and plots it with its counterpart in hemibrain connectome
neuron_to_hemibrain()
#converts a hemibrain neuron into a .nrrd file (to compare to a light-level image image)
hemibrain_to_nrrd()
#converts a specific flywire neuron (from its flywire id) into a .nrrd file (to compare to a light-level image image)
flywireid_to_nrrd()
#writes the cmtk registration command based on the Registration file location
write_cmtkreg()To run the pipeline
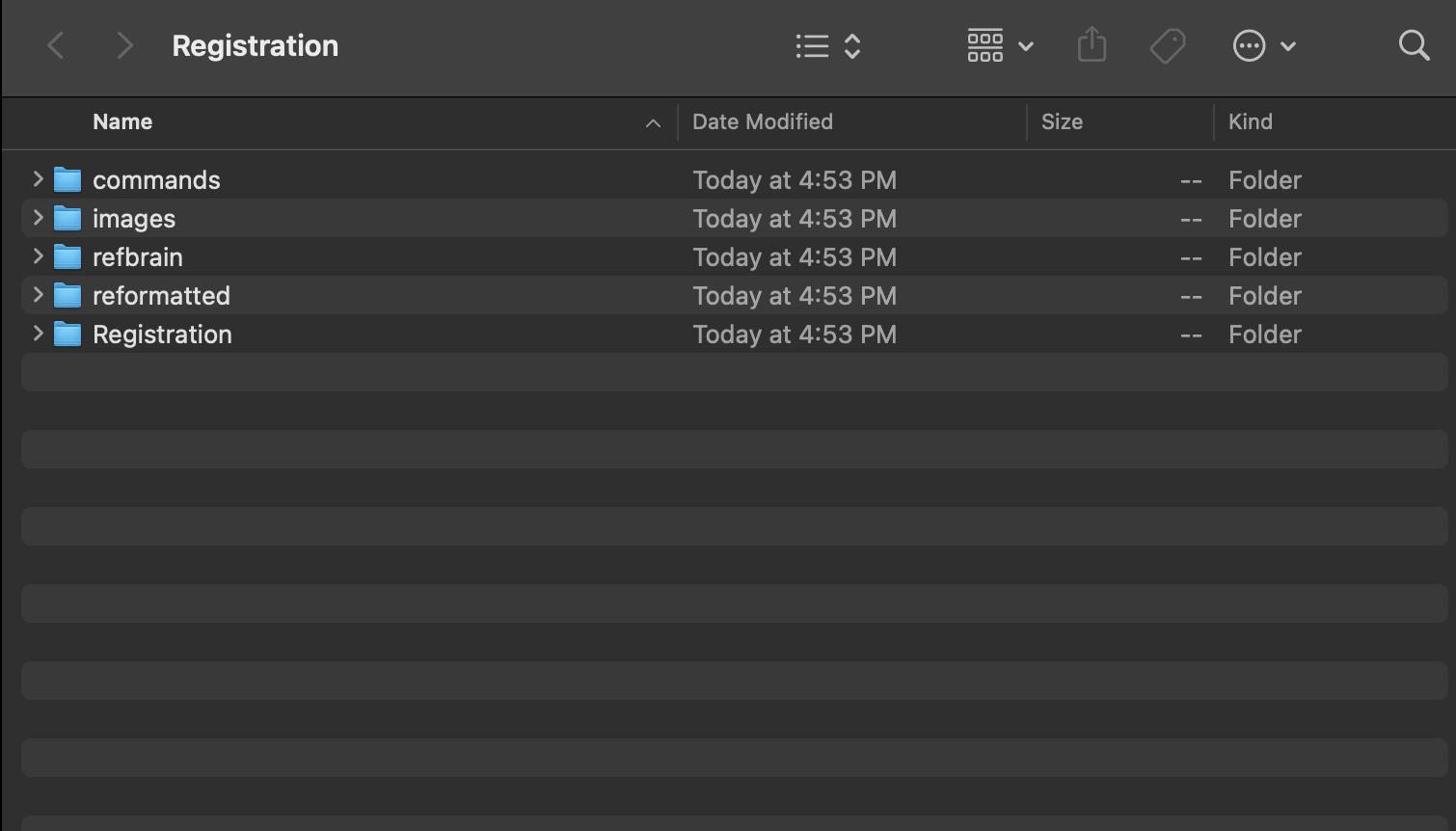
First, install the CMTK gui in FIJI according to the directions on the github page github page and the correct registration folder structure outlined here and below.
Second, go to the parameter.R scripts. Edit the variables to folders
on your local machine. You will also need to edit the 3 lines at the top
of pipeline.R and the top line of functions.R where you will need to
change the paths so that the correct files are sourced correctly.
#this folder is where all of your registration files are, commonly on the desktop
registration_folder = "~/Desktop/Registration"
#this folder is the path where your unprocessed and processed .tif files will be
data_folder = "~/Desktop/to_register"
raw_data = file.path(data_folder,"unprocessed")
processed_data = file.path(data_folder,"processed")
macro1 = "~/nat-tech/R/macros/create_registration_images.ijm"
macro2 = "~/nat-tech/R/macros/create_composite.ijm"
macro3 = "~/nat-tech/R/macros/create_max_projection.ijm"
packages = "~/nat-tech/R/startup/packages.R"
functs = "~/nat-tech/R/startup/functions.R" You will also need to edit the first line at the top of pipeline.R to
source the parameter.R file that you edited above.
# this is the first line of the pipeline code and it ensures that you are sourcing everything correctly
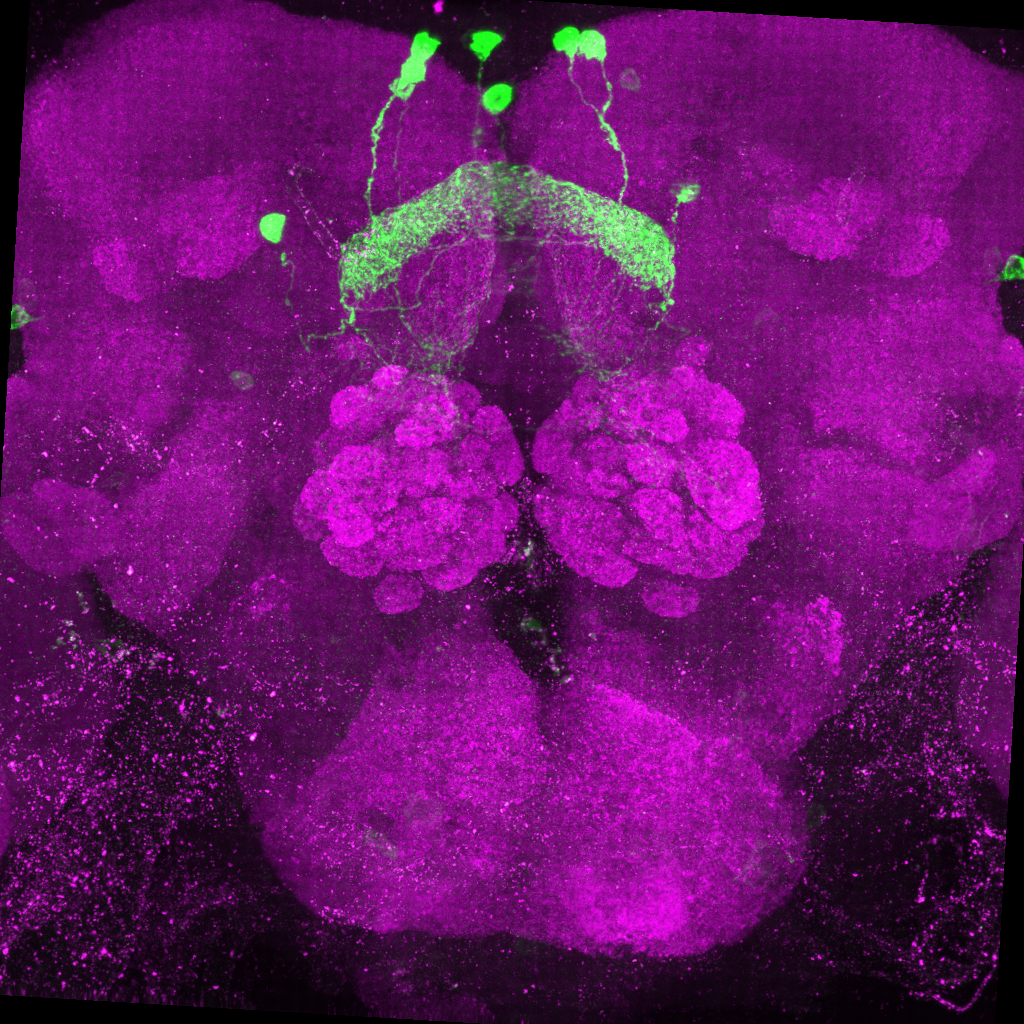
source("~/nat-tech/R/parameters.R")Third, save your 2 channel image as a .tif file (as shown below) in the correct folder, this was specified in the step before. The first channel should be your GFP channel and the second channel should be the channel with the background stain. The way you name this file is also important. The format for a split-gal4 line is date_templatebrain_celltype_AD_GDBD_expnum or for a Gal4 line is date_templatebrain_celltype_Gal4_expnum.
That’s all you have to do! Below is code to run the pipeline in the terminal
$ Rscript /Users/[user]/Documents/GitHub/nat-tech/R/pipeline.RWalkthrough of what’s happening
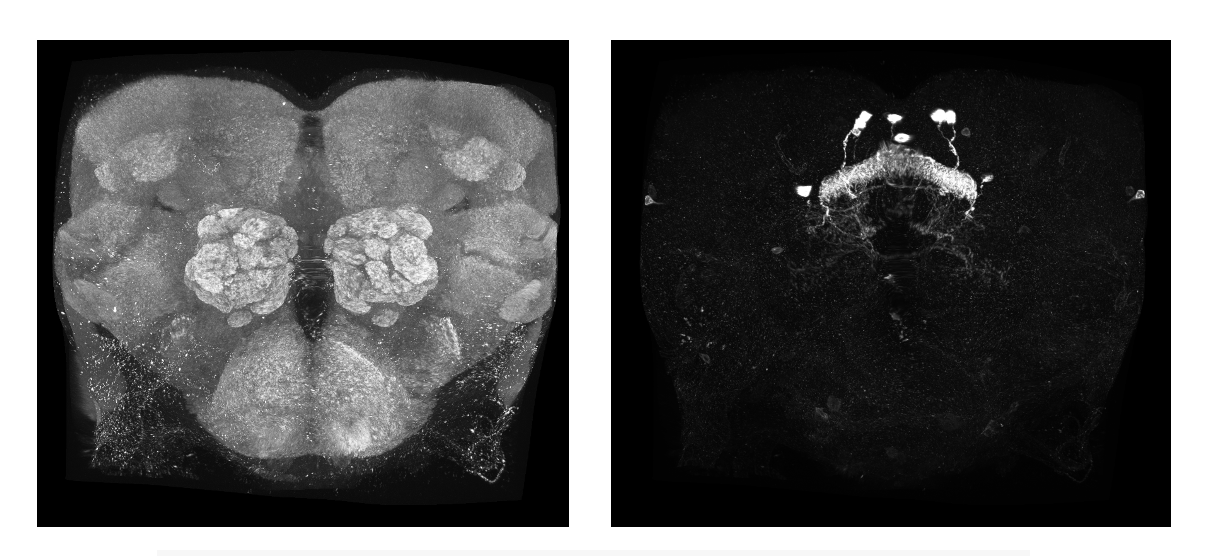
When you start the code, the next thing that will happen is that the code will run a FIJI macro that splits your light level .tif into its respective channels and names them according to the CMTK gui requirements. Then, the program will create the CMTK command file and run that to register and reformat your channels.
runMacro(macro = "R/macros/create_registration_images.ijm")
munger_name = write_cmtkreg()
system(paste0("sh ", munger_name))This is what the reformatted channels should look like.
The next thing that will happen is that the code will get the specified neuron from the hemibrain connectome and then register this to the specified template brain and convert it into a .nrrd file.
hemibrain_to_nrrd()This is what that process looks like
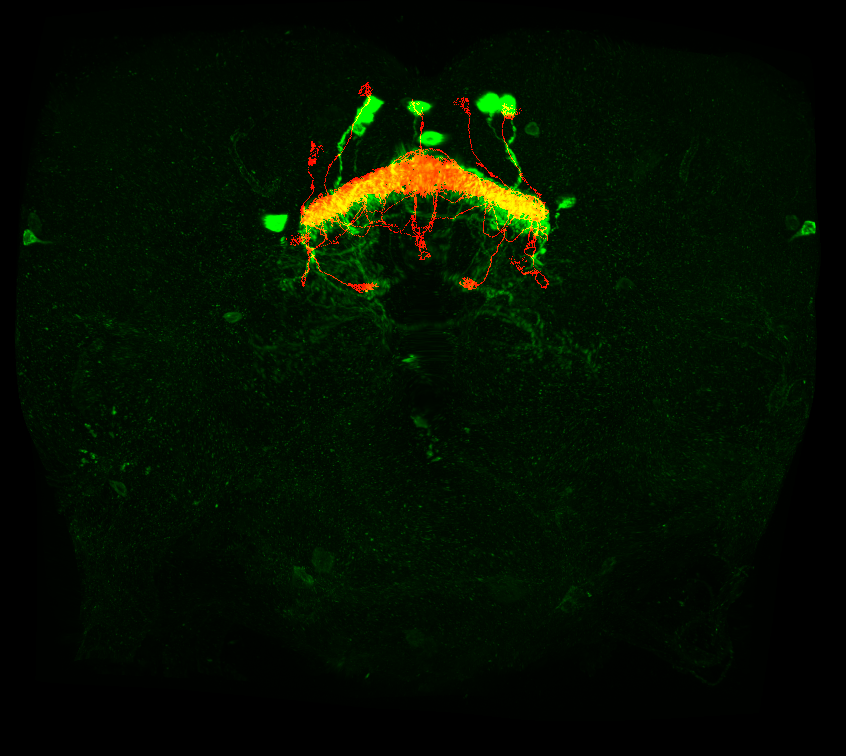
Finally, the code will run two FIJI macros to combine your reformatted images with the specified neuron from the hemibrain that got converted into a .nrrd file and to create a max z projection of that image.
runMacro(macro = "R/macros/create_composite.ijm")
runMacro(macro = "R/macros/create_max_projection.ijm")It should look like this
Things to note
The registration and reformatting process can take a while so you can adjust the number of needed cores in the CMTK command. Below is the line of code you need to edit change in the functions.R script. The part you need to edit is the “-T 4” part where the number is the number of cores. You can use the max number your computer has or as many as you need.
sprintf(\"/Applications/Fiji.app/bin/cmtk/munger\" -b \"/Applications/Fiji.app/bin/cmtk\" -a -w -r 0102 -X 26 -C 8 -G 80 -R 4 -A \"--accuracy 0.4\" -W \"--accuracy 0.4\" -T 4 -s \"Refbrain/%s\" images/%s", template_path, folder)Acknowledgements
This package was created by Emily Kellogg and Alexander Shakeel Bates while in the group of Dr. Rachel Wilson.
Kellogg E and Bates AS (2022). nat-tech R project version 0.1.0. https://github.com/wilson-lab/nat-tech
References
-
The hemibrain connectome (hemibrain:v1.2.1): Scheffer, L.K., Xu, C.S., Januszewski, M., Lu, Z., Takemura, S.-Y., Hayworth, K.J., Huang, G.B., Shinomiya, K., Maitlin-Shepard, J., Berg, S., et al. (2020). A connectome and analysis of the adult Drosophila central brain. Elife 9. doi: https://doi.org/10.7554/eLife.57443
-
JRC2018F brain and VNC templates: Bogovic, J.A., Otsuna, H., Heinrich, L., Ito, M., Jeter, J., Meissner, G.W., Nern, A., Colonell, J., Malkesman, O., Ito, K., et al. (2018). An unbiased template of the Drosophila brain and ventral nerve cord. bioRxiv. doi: https://doi.org/10.1101/376384
-
Computational Morphometry Toolkit (CMTK): Rohlfing T, Maurer CR Jr. Nonrigid image registration in shared-memory multiprocessor environments with application to brains, breasts, and bees. IEEE Trans Inf Technol Biomed. 2003 Mar;7(1):16-25. doi: 10.1109/titb.2003.808506
-
FIJI: Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., … Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nature Methods, 9(7), 676–682. doi:10.1038/nmeth.2019
-
fiji-cmtk-gui: Kohl J, Ostrovsky AD, Frechter S, Jefferis GS. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell. 2013 Dec 19;155(7):1610-23. doi: 10.1016/j.cell.2013.11.025. PMID: 24360281; PMCID: PMC3898676.
-
The natverse: Bates, A.S., Manton, J.D., Jagannathan, S.R., Costa, M., Schlegel, P., Rohlfing, T., Jefferis, G.(2020) The natverse, a versatile toolbox for combining and analysing neuroanatomical data eLife 9:e53350. doi: https://doi.org/10.7554/eLife.53350 )
-
FlyWire: Dorkenwald, S., McKellar, C.E., Macrina, T. et al. FlyWire: online community for whole-brain connectomics. Nat Methods 19, 119–128 (2022).doi: https://doi.org/10.1038/s41592-021-01330-0