This package provides an implementation of the inference pipeline of AlphaFold v2.0. This is a completely new model that was entered in CASP14 and published in Nature. For simplicity, we refer to this model as AlphaFold throughout the rest of this document.
We also provide an implementation of AlphaFold-Multimer. This represents a work in progress and AlphaFold-Multimer isn't expected to be as stable as our monomer AlphaFold system.
Any publication that discloses findings arising from using this source code or the model parameters should cite the AlphaFold paper and, if applicable, the AlphaFold-Multimer paper.
Please also refer to the Supplementary Information for a detailed description of the method.
You can use a slightly simplified version of AlphaFold with this Colab notebook or community-supported versions (see below).
The following steps are required in order to run AlphaFold:
- Download genetic databases (see below).
- Download model parameters (see below).
This step requires aria2c to be installed on your machine.
AlphaFold needs multiple genetic (sequence) databases to run:
- BFD,
- MGnify,
- PDB70,
- PDB (structures in the mmCIF format),
- PDB seqres – only for AlphaFold-Multimer,
- Uniclust30,
- UniProt – only for AlphaFold-Multimer,
- UniRef90.
We provide a script scripts/download_all_data.sh that can be used to download
and set up all of these databases:
-
Default:
scripts/download_all_data.sh <DOWNLOAD_DIR>
will download the full databases.
-
With
reduced_dbs:scripts/download_all_data.sh <DOWNLOAD_DIR> reduced_dbs
will download a reduced version of the databases to be used with the
reduced_dbsdatabase preset.
We don't provide exactly the database versions used in CASP14 – see the note on reproducibility. Some of the databases are mirrored for speed, see mirrored databases.
📒 Note: The total download size for the full databases is around 415 GB and the total size when unzipped is 2.2 TB. Please make sure you have a large enough hard drive space, bandwidth and time to download. We recommend using an SSD for better genetic search performance.
The download_all_data.sh script will also download the model parameter files.
Once the script has finished, you should have the following directory structure:
$DOWNLOAD_DIR/ # Total: ~ 2.2 TB (download: 438 GB)
bfd/ # ~ 1.7 TB (download: 271.6 GB)
# 6 files.
mgnify/ # ~ 64 GB (download: 32.9 GB)
mgy_clusters_2018_12.fa
params/ # ~ 3.5 GB (download: 3.5 GB)
# 5 CASP14 models,
# 5 pTM models,
# 5 AlphaFold-Multimer models,
# LICENSE,
# = 16 files.
pdb70/ # ~ 56 GB (download: 19.5 GB)
# 9 files.
pdb_mmcif/ # ~ 206 GB (download: 46 GB)
mmcif_files/
# About 180,000 .cif files.
obsolete.dat
pdb_seqres/ # ~ 0.2 GB (download: 0.2 GB)
pdb_seqres.txt
small_bfd/ # ~ 17 GB (download: 9.6 GB)
bfd-first_non_consensus_sequences.fasta
uniclust30/ # ~ 86 GB (download: 24.9 GB)
uniclust30_2018_08/
# 13 files.
uniprot/ # ~ 98.3 GB (download: 49 GB)
uniprot.fasta
uniref90/ # ~ 58 GB (download: 29.7 GB)
uniref90.fasta
bfd/ is only downloaded if you download the full databases, and small_bfd/
is only downloaded if you download the reduced databases.
While the AlphaFold code is licensed under the Apache 2.0 License, the AlphaFold parameters are made available under the terms of the CC BY 4.0 license. Please see the Disclaimer below for more detail.
The AlphaFold parameters are available from
https://storage.googleapis.com/alphafold/alphafold_params_2022-01-19.tar, and
are downloaded as part of the scripts/download_all_data.sh script. This script
will download parameters for:
- 5 models which were used during CASP14, and were extensively validated for structure prediction quality (see Jumper et al. 2021, Suppl. Methods 1.12 for details).
- 5 pTM models, which were fine-tuned to produce pTM (predicted TM-score) and (PAE) predicted aligned error values alongside their structure predictions (see Jumper et al. 2021, Suppl. Methods 1.9.7 for details).
- 5 AlphaFold-Multimer models that produce pTM and PAE values alongside their structure predictions.
-
Install Miniconda:
# Retrieve Miniconda: wget https://repo.anaconda.com/miniconda/Miniconda3-latest-Linux-x86_64.sh # Install Miniconda: bash Miniconda3-latest-Linux-x86_64.sh
- Remaining instructions assume Miniconda is now installed to '~/miniconda3'
-
Install and configure Conda environment:
# Clone this repository: git clone https://github.com/amorehead/alphafold_multimer_non_docker # Change to project directory: cd alphafold_multimer_non_docker AF2_DIR=$(pwd) # Set up Conda environment locally: conda env create --name alphafold_non_docker -f environment.yml # Update Conda post-install: conda update -n base conda # Activate Conda environment located in the current directory: conda activate alphafold_non_docker # (Optional) Perform a full install of the pip dependencies described in 'requirements.txt': pip3 install -r requirements.txt # (Optional) To remove the long Conda environment prefix in your shell prompt, modify the env_prompt setting in your .condarc file with: conda config --set env_prompt '({name})'
-
Download chemical properties to common directory:
wget -q -P "$AF2_DIR"/alphafold/common/ https://git.scicore.unibas.ch/schwede/openstructure/-/raw/7102c63615b64735c4941278d92b554ec94415f8/modules/mol/alg/src/stereo_chemical_props.txt -
Apply OpenMM patch:
cd ~/miniconda3/envs/alphafold_non_docker/lib/python3.8/site-packages/ && patch -p0 < "$AF2_DIR"/patches/openmm.patch
-
Reference our custom Bash script to run AlphaFold:
Usage: run_alphafold.sh <OPTIONS> Required Parameters: -d <data_dir> Path to directory with supporting data: AlphaFold parameters and genetic and template databases. Set to the target of download_all_databases.sh. -o <output_dir> Path to a directory that will store the results. -f <fasta_path> Path to a FASTA file containing a single sequence. -t <max_template_date> Maximum template release date to consider (ISO-8601 format: YYYY-MM-DD). Important if folding historical test sets. Optional Parameters: -n <openmm_threads> OpenMM threads (default: all available cores) -b <benchmark> Run multiple JAX model evaluations to obtain a timing that excludes the compilation time, which should be more indicative of the time required for inferencing many proteins (default: false) -g <use_gpu> Enable NVIDIA runtime to run with GPUs (default: true) -a <gpu_devices> Comma separated list of devices to pass to 'CUDA_VISIBLE_DEVICES' (default: 0) -m <model_preset> Choose preset model configuration - the monomer model (monomer), the monomer model with extra ensembling (monomer_casp14), monomer model with pTM head (monomer_ptm), or multimer model (multimer) (default: monomer) -p <db_preset> Choose preset MSA database configuration - smaller genetic database config (reduced_dbs) or full genetic database config (full_dbs) (default: full_dbs) -u <use_precomputed_msas> Whether to read MSAs that have been written to disk. WARNING: This will not check if the sequence, database or configuration have changed. (default: false) -r <remove_msas_after_use> Whether, after structure prediction(s), to delete MSAs that have been written to disk to significantly free up storage space. (default: false) -i <is_prokaryote> Optional for multimer system, not used by the single chain system. This should contain a boolean specifying true where the target complex is from a prokaryote, and false where it is not, or where the origin is unknown. These values determine the pairing method for the MSA (default: false)
-
Run
run_alphafold.shpointing to a FASTA file containing the protein sequence(s) for which you wish to predict the structure. If you are predicting the structure of a protein that is already in PDB and you wish to avoid using it as a template, thenmax_template_datemust be set to be before the release date of the structure. You must also provide the path to the directory containing the downloaded databases. For example, for the T1050 CASP14 target:bash run_alphafold.sh \ -d $DOWNLOAD_DIR \ -o $OUTPUT_DIR \ -f T1050.fasta \ -t 2020-05-14
By default, Alphafold will attempt to use the first visible GPU device. To use a subset, specify a comma-separated list of GPU UUID(s) or index(es) using the
--gpu_devicesflag. See GPU enumeration for more details. -
You can control which AlphaFold model to run by adding the
--model_preset=flag. We provide the following models:-
monomer: This is the original model used at CASP14 with no ensembling.
-
monomer_casp14: This is the original model used at CASP14 with
num_ensemble=8, matching our CASP14 configuration. This is largely provided for reproducibility as it is 8x more computationally expensive for limited accuracy gain (+0.1 average GDT gain on CASP14 domains). -
monomer_ptm: This is the original CASP14 model fine tuned with the pTM head, providing a pairwise confidence measure. It is slightly less accurate than the normal monomer model.
-
multimer: This is the AlphaFold-Multimer model. To use this model, provide a multi-sequence FASTA file. In addition, the UniProt database should have been downloaded.
-
-
You can control MSA speed/quality tradeoff by adding
--db_preset=reduced_dbsor--db_preset=full_dbsto the run command. We provide the following presets:-
reduced_dbs: This preset is optimized for speed and lower hardware requirements. It runs with a reduced version of the BFD database. It requires 8 CPU cores (vCPUs), 8 GB of RAM, and 600 GB of disk space.
-
full_dbs: This runs with all genetic databases used at CASP14.
Running the command above with the
monomermodel preset and thereduced_dbsdata preset would look like this:bash run_alphafold.sh \ -d $DOWNLOAD_DIR \ -o $OUTPUT_DIR \ -f T1050.fasta \ -t 2020-05-14 \ -m monomer \ -p reduced_dbs
-
All steps are the same as when running the monomer system, but you will have to
- provide an input fasta with multiple sequences,
- set
--model_preset=multimer, - optionally set the
--is_prokaryoteflag with a boolean that determines whether the input sequence in the given fasta file is prokaryotic. If that is not the case or the origin is unknown, set tofalsefor the fasta.
An example that folds a protein complex multimer.fasta that is prokaryotic:
bash run_alphafold.sh \
-d $DOWNLOAD_DIR \
-o $OUTPUT_DIR \
-f multimer.fasta \
-t 2020-05-14 \
-m multimer \
-i trueBelow are examples on how to use AlphaFold in different scenarios.
Say we have a monomer with the sequence <SEQUENCE>. The input fasta should be:
>sequence_name
<SEQUENCE>
Then run the following command:
bash run_alphafold.sh \
-d $DOWNLOAD_DIR \
-o $OUTPUT_DIR \
-f monomer.fasta \
-t 2021-11-01 \
-m monomerSay we have a homomer from a prokaryote with 3 copies of the same sequence
<SEQUENCE>. The input fasta should be:
>sequence_1
<SEQUENCE>
>sequence_2
<SEQUENCE>
>sequence_3
<SEQUENCE>
Then run the following command:
bash run_alphafold.sh \
-d $DOWNLOAD_DIR \
-o $OUTPUT_DIR \
-f homomer.fasta \
-t 2021-11-01 \
-m multimer \
-i trueSay we have a heteromer A2B3 of unknown origin, i.e. with 2 copies of
<SEQUENCE A> and 3 copies of <SEQUENCE B>. The input fasta should be:
>sequence_1
<SEQUENCE A>
>sequence_2
<SEQUENCE A>
>sequence_3
<SEQUENCE B>
>sequence_4
<SEQUENCE B>
>sequence_5
<SEQUENCE B>
Then run the following command:
bash run_alphafold.sh \
-d $DOWNLOAD_DIR \
-o $OUTPUT_DIR \
-f heteromer.fasta \
-t 2021-11-01 \
-m multimer \
-i falseThe outputs will be saved in a subdirectory of the directory provided via the
--output_dir flag of run_alphafold.sh. The outputs include the computed MSAs,
unrelaxed structures, relaxed structures, ranked structures, raw model outputs,
prediction metadata, and section timings.
The --output_dir directory will have the following structure:
<target_name>/
features.pkl
ranked_{0,1,2,3,4}.pdb
ranking_debug.json
relaxed_model_{1,2,3,4,5}.pdb
result_model_{1,2,3,4,5}.pkl
timings.json
unrelaxed_model_{1,2,3,4,5}.pdb
msas/
bfd_uniclust_hits.a3m
mgnify_hits.sto
uniref90_hits.sto
The contents of each output file are as follows:
-
features.pkl– Apicklefile containing the input feature NumPy arrays used by the models to produce the structures. -
unrelaxed_model_*.pdb– A PDB format text file containing the predicted structure, exactly as outputted by the model. -
relaxed_model_*.pdb– A PDB format text file containing the predicted structure, after performing an Amber relaxation procedure on the unrelaxed structure prediction (see Jumper et al. 2021, Suppl. Methods 1.8.6 for details). -
ranked_*.pdb– A PDB format text file containing the relaxed predicted structures, after reordering by model confidence. Hereranked_0.pdbshould contain the prediction with the highest confidence, andranked_4.pdbthe prediction with the lowest confidence. To rank model confidence, we use predicted LDDT (pLDDT) scores (see Jumper et al. 2021, Suppl. Methods 1.9.6 for details). -
ranking_debug.json– A JSON format text file containing the pLDDT values used to perform the model ranking, and a mapping back to the original model names. -
timings.json– A JSON format text file containing the times taken to run each section of the AlphaFold pipeline. -
msas/- A directory containing the files describing the various genetic tool hits that were used to construct the input MSA. -
result_model_*.pkl– Apicklefile containing a nested dictionary of the various NumPy arrays directly produced by the model. In addition to the output of the structure module, this includes auxiliary outputs such as:- Distograms (
distogram/logitscontains a NumPy array of shape [N_res, N_res, N_bins] anddistogram/bin_edgescontains the definition of the bins). - Per-residue pLDDT scores (
plddtcontains a NumPy array of shape [N_res] with the range of possible values from0to100, where100means most confident). This can serve to identify sequence regions predicted with high confidence or as an overall per-target confidence score when averaged across residues. - Present only if using pTM models: predicted TM-score (
ptmfield contains a scalar). As a predictor of a global superposition metric, this score is designed to also assess whether the model is confident in the overall domain packing. - Present only if using pTM models: predicted pairwise aligned errors
(
predicted_aligned_errorcontains a NumPy array of shape [N_res, N_res] with the range of possible values from0tomax_predicted_aligned_error, where0means most confident). This can serve for a visualisation of domain packing confidence within the structure.
- Distograms (
The pLDDT confidence measure is stored in the B-factor field of the output PDB files (although unlike a B-factor, higher pLDDT is better, so care must be taken when using for tasks such as molecular replacement).
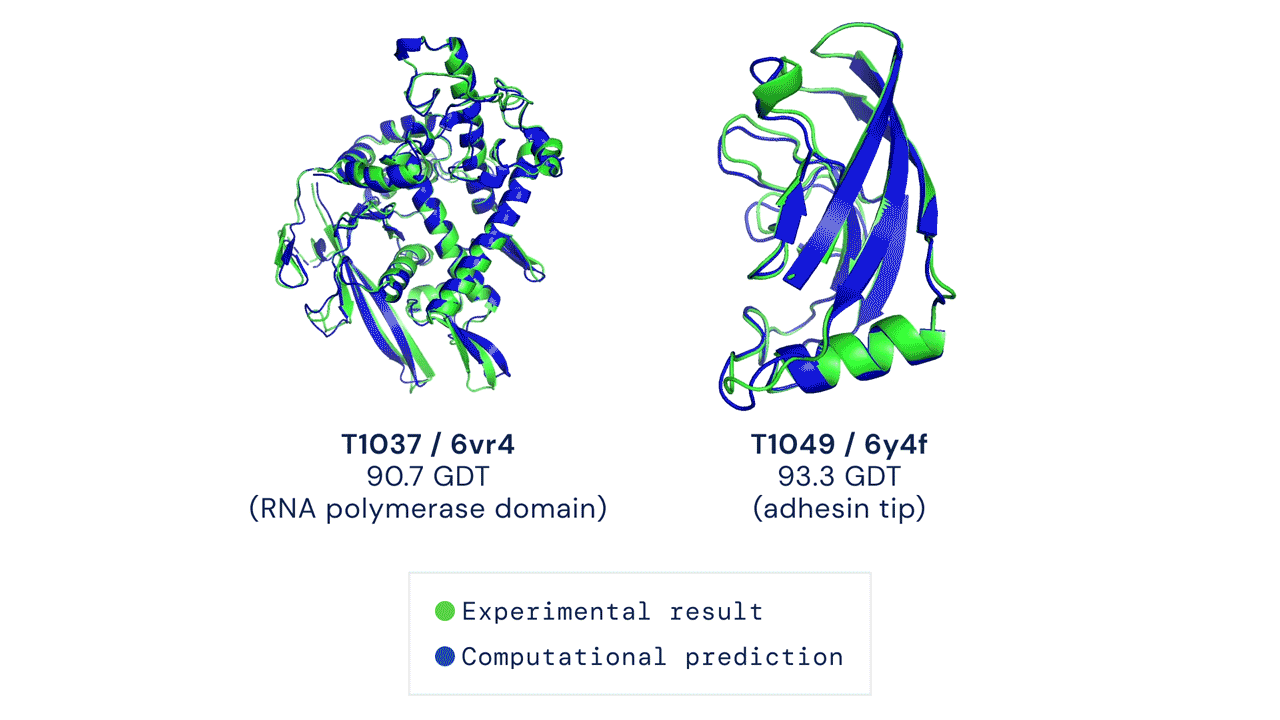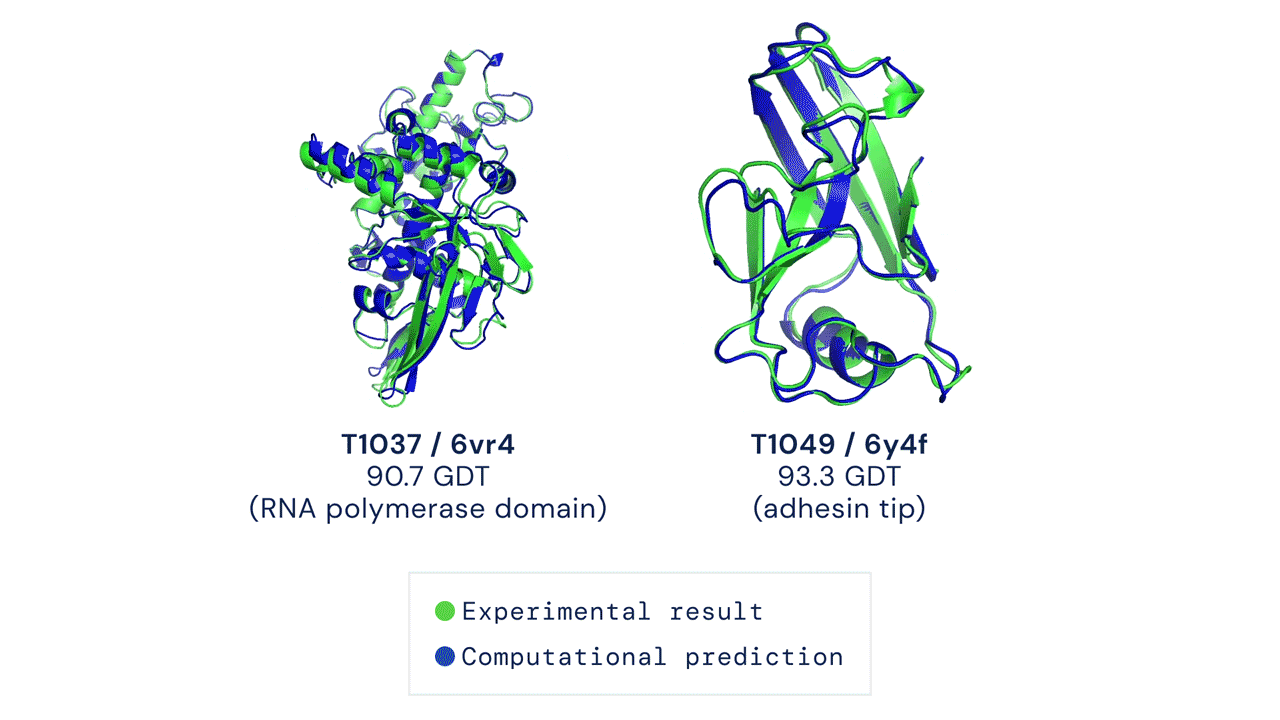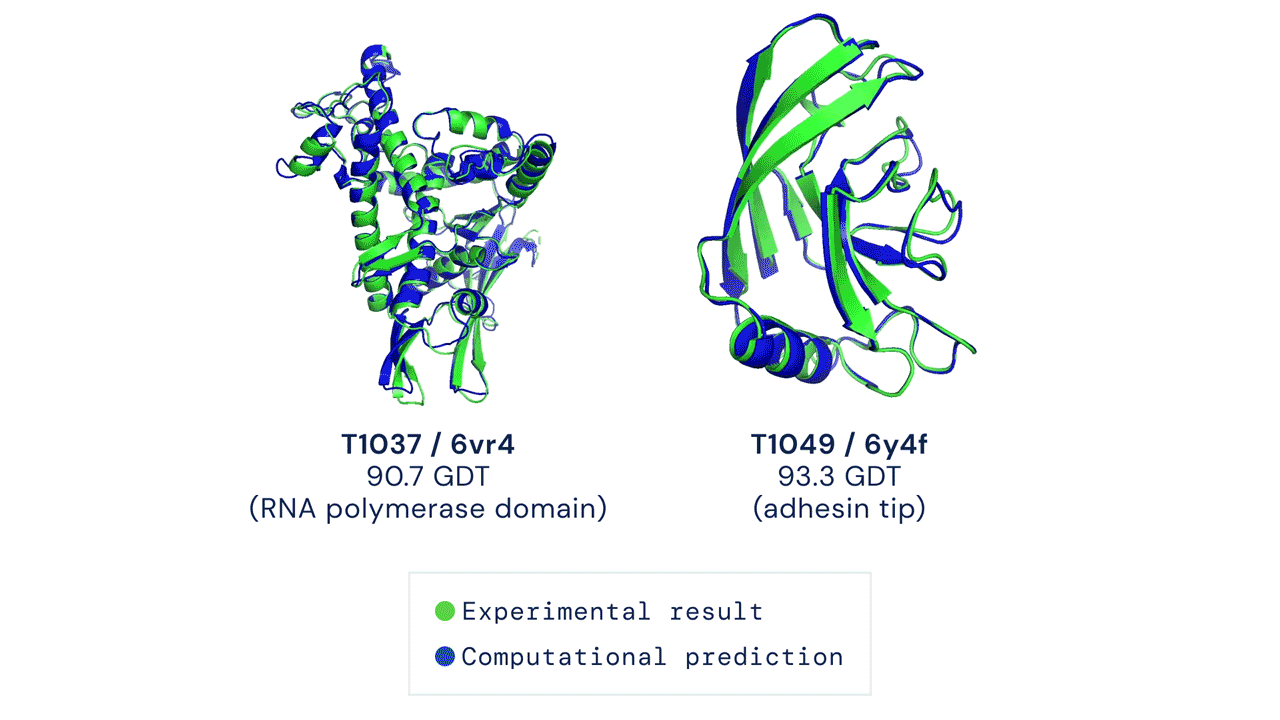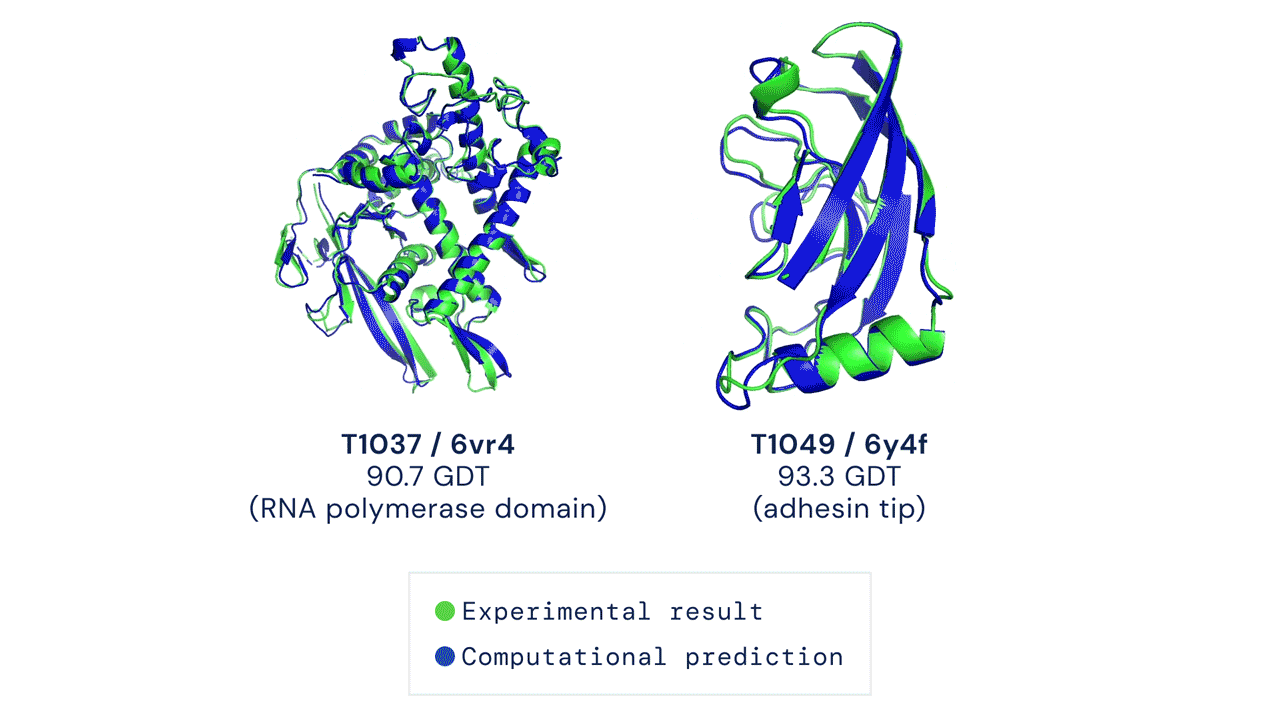
This code has been tested to match mean top-1 accuracy on a CASP14 test set with pLDDT ranking over 5 model predictions (some CASP targets were run with earlier versions of AlphaFold and some had manual interventions; see our forthcoming publication for details). Some targets such as T1064 may also have high individual run variance over random seeds.
The provided inference script is optimized for predicting the structure of a
single protein, and it will compile the neural network to be specialized to
exactly the size of the sequence, MSA, and templates. For large proteins, the
compile time is a negligible fraction of the runtime, but it may become more
significant for small proteins or if the multi-sequence alignments are already
precomputed. In the bulk inference case, it may make sense to use our
make_fixed_size function to pad the inputs to a uniform size, thereby reducing
the number of compilations required.
We do not provide a bulk inference script, but it should be straightforward to
develop on top of the RunModel.predict method with a parallel system for
precomputing multi-sequence alignments. Alternatively, this script can be run
repeatedly with only moderate overhead.
AlphaFold's output for a small number of proteins has high inter-run variance, and may be affected by changes in the input data. The CASP14 target T1064 is a notable example; the large number of SARS-CoV-2-related sequences recently deposited changes its MSA significantly. This variability is somewhat mitigated by the model selection process; running 5 models and taking the most confident.
To reproduce the results of our CASP14 system as closely as possible you must use the same database versions we used in CASP. These may not match the default versions downloaded by our scripts.
For genetics:
- UniRef90: v2020_01
- MGnify: v2018_12
- Uniclust30: v2018_08
- BFD: only version available
For templates:
- PDB: (downloaded 2020-05-14)
- PDB70: 2020-05-13
An alternative for templates is to use the latest PDB and PDB70, but pass the
flag --max_template_date=2020-05-14, which restricts templates only to
structures that were available at the start of CASP14.
If you use the code or data in this package, please cite:
@Article{AlphaFold2021,
author = {Jumper, John and Evans, Richard and Pritzel, Alexander and Green, Tim and Figurnov, Michael and Ronneberger, Olaf and Tunyasuvunakool, Kathryn and Bates, Russ and {\v{Z}}{\'\i}dek, Augustin and Potapenko, Anna and Bridgland, Alex and Meyer, Clemens and Kohl, Simon A A and Ballard, Andrew J and Cowie, Andrew and Romera-Paredes, Bernardino and Nikolov, Stanislav and Jain, Rishub and Adler, Jonas and Back, Trevor and Petersen, Stig and Reiman, David and Clancy, Ellen and Zielinski, Michal and Steinegger, Martin and Pacholska, Michalina and Berghammer, Tamas and Bodenstein, Sebastian and Silver, David and Vinyals, Oriol and Senior, Andrew W and Kavukcuoglu, Koray and Kohli, Pushmeet and Hassabis, Demis},
journal = {Nature},
title = {Highly accurate protein structure prediction with {AlphaFold}},
year = {2021},
volume = {596},
number = {7873},
pages = {583--589},
doi = {10.1038/s41586-021-03819-2}
}In addition, if you use the AlphaFold-Multimer mode, please cite:
@article {AlphaFold-Multimer2021,
author = {Evans, Richard and O{\textquoteright}Neill, Michael and Pritzel, Alexander and Antropova, Natasha and Senior, Andrew and Green, Tim and {\v{Z}}{\'\i}dek, Augustin and Bates, Russ and Blackwell, Sam and Yim, Jason and Ronneberger, Olaf and Bodenstein, Sebastian and Zielinski, Michal and Bridgland, Alex and Potapenko, Anna and Cowie, Andrew and Tunyasuvunakool, Kathryn and Jain, Rishub and Clancy, Ellen and Kohli, Pushmeet and Jumper, John and Hassabis, Demis},
journal = {bioRxiv},
title = {Protein complex prediction with AlphaFold-Multimer},
year = {2021},
elocation-id = {2021.10.04.463034},
doi = {10.1101/2021.10.04.463034},
URL = {https://www.biorxiv.org/content/early/2021/10/04/2021.10.04.463034},
eprint = {https://www.biorxiv.org/content/early/2021/10/04/2021.10.04.463034.full.pdf},
}Colab notebooks provided by the community (please note that these notebooks may vary from our full AlphaFold system and we did not validate their accuracy):
- The ColabFold AlphaFold2 notebook by Martin Steinegger, Sergey Ovchinnikov and Milot Mirdita, which uses an API hosted at the Södinglab based on the MMseqs2 server (Mirdita et al. 2019, Bioinformatics) for the multiple sequence alignment creation.
AlphaFold communicates with and/or references the following separate libraries and packages:
- Abseil
- Biopython
- Chex
- Colab
- HH Suite
- HMMER Suite
- Haiku
- Immutabledict
- JAX
- Kalign
- matplotlib
- ML Collections
- NumPy
- OpenMM
- OpenStructure
- pandas
- pymol3d
- SciPy
- Sonnet
- TensorFlow
- Tree
- tqdm
We thank all their contributors and maintainers!
This is not an officially supported Google product.
Copyright 2021 DeepMind Technologies Limited.
Licensed under the Apache License, Version 2.0 (the "License"); you may not use this file except in compliance with the License. You may obtain a copy of the License at https://www.apache.org/licenses/LICENSE-2.0.
Unless required by applicable law or agreed to in writing, software distributed under the License is distributed on an "AS IS" BASIS, WITHOUT WARRANTIES OR CONDITIONS OF ANY KIND, either express or implied. See the License for the specific language governing permissions and limitations under the License.
The AlphaFold parameters are made available under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) license. You can find details at: https://creativecommons.org/licenses/by/4.0/legalcode
Use of the third-party software, libraries or code referred to in the Acknowledgements section above may be governed by separate terms and conditions or license provisions. Your use of the third-party software, libraries or code is subject to any such terms and you should check that you can comply with any applicable restrictions or terms and conditions before use.
The following databases have been mirrored by DeepMind, and are available with reference to the following:
-
BFD (unmodified), by Steinegger M. and Söding J., available under a Creative Commons Attribution-ShareAlike 4.0 International License.
-
BFD (modified), by Steinegger M. and Söding J., modified by DeepMind, available under a Creative Commons Attribution-ShareAlike 4.0 International License. See the Methods section of the AlphaFold proteome paper for details.
-
Uniclust30: v2018_08 (unmodified), by Mirdita M. et al., available under a Creative Commons Attribution-ShareAlike 4.0 International License.
-
MGnify: v2018_12 (unmodified), by Mitchell AL et al., available free of all copyright restrictions and made fully and freely available for both non-commercial and commercial use under CC0 1.0 Universal (CC0 1.0) Public Domain Dedication.