quantum
An easy-to-use quantum mechanics library with a focus on visualization.
Preface
This project is very much inspired by the stunning visualization by Henry Reich. They show bohmian trajectories of electrons in hydrogen-like atoms. But sadly no source code was published. Thus the initial goal of this project was to recreate these visualizations, that is implement the quantum wave function for hydrogen-like atoms and generate the bohmian trajectories from it. But why stop there.
Please note that the overall aim was still of educational nature, i.e. the code is ment to be easy-to-use but also easy-to-read. Thus implementations are not the fastest. However, where possible, algorithms are implemented in a vectorized manner allowing for fast tensor operations using numpy and scipy.
Requirements
All algorithms and routines are implemented in python using the following dependencies
- numpy >= 1.21.5
- scipy >= 1.7.3
However other modules (matplotlib, etc.) are used in the examples for visualization. The dependencies can be installed by the following command
python -m pip install -f requirements.txtModules
The library is separated into the following modular components:
| Module | Desciption |
|---|---|
| core | Holds core classes including the WaveFunction and TISE. |
| analytic | Contains analytic solutions to the schrödinger equation for well-known potentials. This includes the HydrogenLike wave funciton. |
| chemistry | Holds quantum chemistry components providing functionality to approximate molecular orbitals. Main classes are the ElectronicTISE and GaussianOrbital. |
| extra | Extra functionality building on top of but is not used within the library. E.g. the BohmianMechanics class is part of this module. |
| utils | Utilities used throughout the library. |
Examples
Several examples showcasing the current state of the quantum library can be found in the example directory. Some of them are covered below.
Solving the Time-Indipendent Schrödinger Equation
To solve the time indipendent schrödinger equation one must first define the potential energy function. The function must accept a numpy array of shape (..., ndim) where ndim specifies the dimensionality. The following snipped defined the potential energy for a n-dimensional harmonic oscillator:
V = lambda x: 0.5 * (x**2).sum(axis=-1)
# or using the extra.potentials module
V = quantum.extra.potentials.HarmonicOscillatorPotential(w=1.0)Note that some well-known potentials are defined in the extra.potentials module.
Now solving the time-indipendent schrödinger equation for the 2-dimensional harmonic oscillator is as easy as (from examples/harmonic_oscillator.py):
eq = quantum.TISE(V)
# solve the equation and return a list of found stable
# states/wave-functions
states = eq.solve(
# specify the boundary of the grid on which to solve
# the schroedinger equation. make sure that the bounds
# are chosen such that the boundary conditions are met,
# i.e. psi(-inf) = psi(inf) = 0
bounds=[[-10, 10], [-10, 10]],
# the distance between points on the regular grid
dx=0.1,
# the number of eigenstates to solve for
# by default will search for the k states with the
# smallest eigenvalues/energies
k=2
)Note that the dimensionality is determined by the bounds argument, i.e. it specifies the lower and upper bound for each dimension.
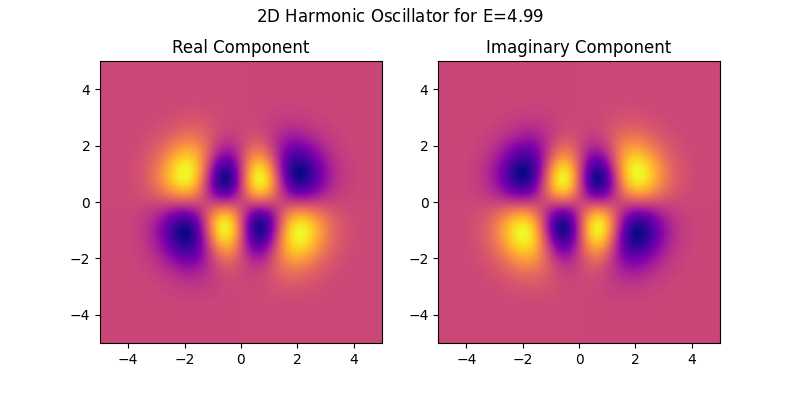
The following visualization shows the real and complex components of a wave function for a specific energy value that was generated by the above (from examples/harmonic_oscillator.py).
Superposition: Interference Pattern
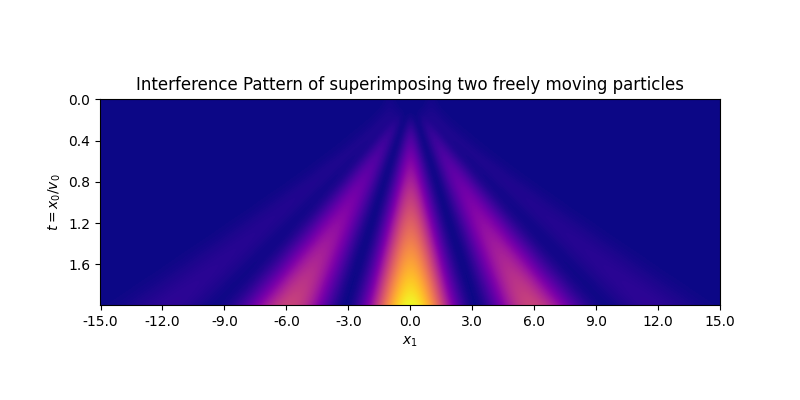
A somewhat more involved example is to generate the interference pattern by superimposing free-moving particles. A freely moving particle can be represented by a gaussian wave packet which has an analytic form. The following code snippet creates a wave function that descibes a single particle in the setup of the double slit experiment (from examples/interference.py):
wf = quantum.analytic.GaussianWavePacket(v=[0.1, 0.0], x0=[0.0, 1.0], s0=0.2) + \
quantum.analytic.GaussianWavePacket(v=[0.1, 0.0], x0=[0.0, -1.0], s0=0.2)A simple visualization of the resulting wave function already shows the interference pattern (from examples/interference.py)
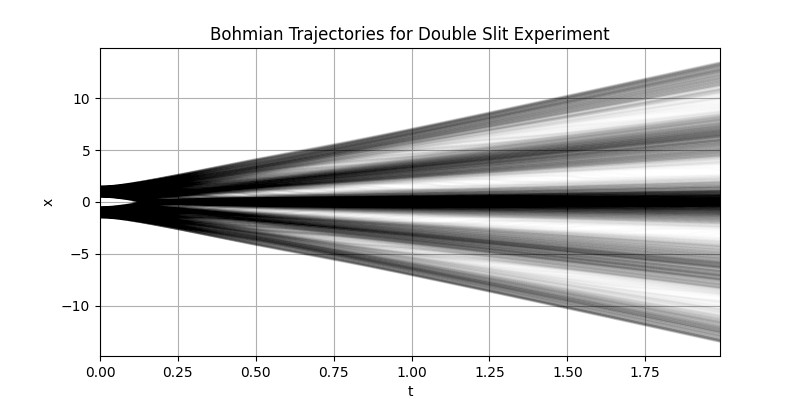
De-Boglie-Bohm Theory: Interference Pattern
Inspired by this work of Dane Odekirk one can also compute the bohmian trajectories of particles starting out in one of the two slits. First one needs to generate the initial positions of the particles (from examples/interference.py):
# create initial particle positions within slits
q = np.random.uniform(-1, 1, size=(num_particles, 1))
q = np.concatenate((
np.zeros_like(q),
1.0 * q + 0.5 * np.sign(q)
), axis=-1)Then the trajectories can be computed using the BohmianMechanics class as follows:
# time steps at which to evaluate for their trajectories
t = np.arange(0, 2, 0.01)
# compute bohmian trajectories
Q = quantum.extra.BohmianMechanics(wave=wf).trajectory(q=q, t=t)Visualizing the trajectories yields (from examples/interference.py). Note that in comparison to the above interference pattern, here the axes are swapped.
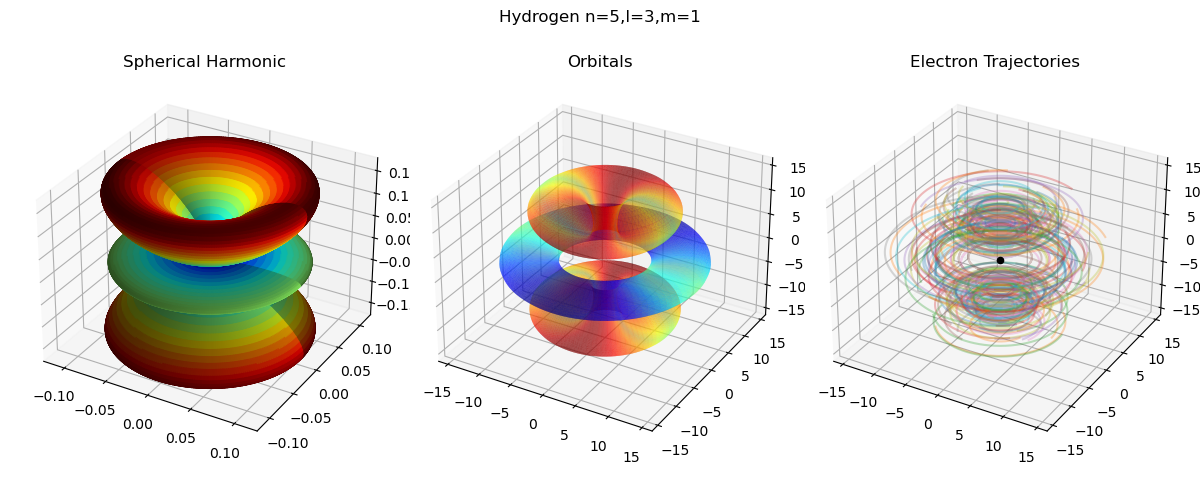
Hydrogen-Like Atoms
As seen in the above example, the bohmian trajectories can be computed for arbitrary wave functions. Thus one can also generate the bohmian electron trajectories within hydrogen-like atoms. The corresponding wave function can be created by (from examples/hydrogen.py):
wf = quantum.analytic.HydrogenLike(5, 3, 1)The easiest way to create initial positions for the trajectories is to simply sample points on the probability density function implied by the wave function. This can be done by using multi-dimensional inverse transfer sampling as follows (from examples/hydrogen.py):
# sample points from probability density at time t=0
s = quantum.utils.sampling.inverse_transfer_sampling(
pdf=partial(wf.pdf, t=0),
num_samples=50000,
bounds=[[0, 8], [0, 2*np.pi], [0, np.pi]],
delta=0.05
)Plugging this into the BohmianMechanics class and visualizing the corresponding trajectories gives the most right plot in the following figure (from examples/hydrogen.py)
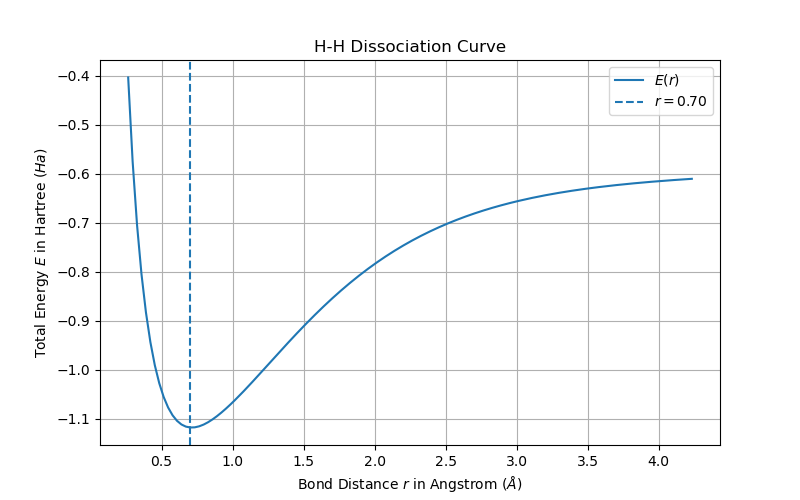
Bond Dissociation Curves of Molecules
After working with one-electron atoms the next step is to consider molecules. There is no analytic solution to the schrödinger equation of molecules. However with a fair amount of approximations the electronic schrödinger equation can be solved numerically. A first approximation is the desciption of atoms. Here an atom is typically described by a set of Gaussian Type Orbitals (GTOs), which together approximate the electron orbitals of the atom. The following code snippet defines two hydrogen atoms with different origins in the STO-3G basis using the API provided by BasisSetExchange:
# hydrogen 1s orbital in STO-3G basis taken from basis-set-exchange
H_1 = Atom.from_BSE(basis="STO-3G", element="H")
H_2 = Atom.from_BSE(basis="STO-3G", element="H", origin=[0, 0, 1.4])A molecule then is decribed by the union of all the GTOs of its atoms together with the atom positions and nuclei charges. Putting all this information togther, the corresponding electronic schrödinger equation can be created and solved as follows:
tise = ElectronicTISE(H_1 + H_2)
# solve using restricted hartree fock
E, MOs = tise.restricted_hartree_fock()Repeating the above for different atomic distances and plotting the result leads to the following figure (from examples/hartree_fock.py):
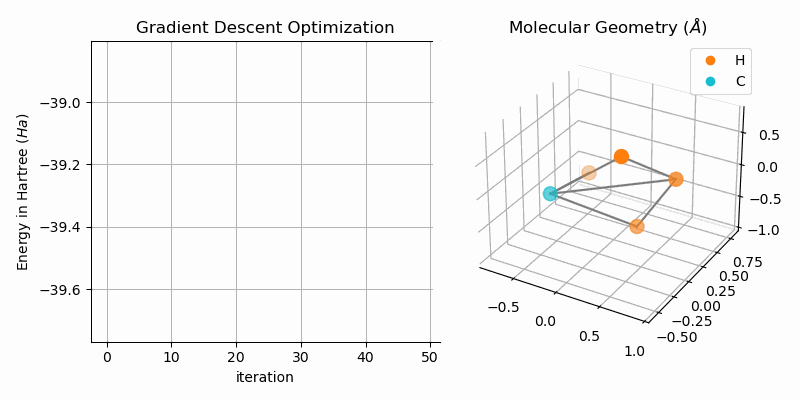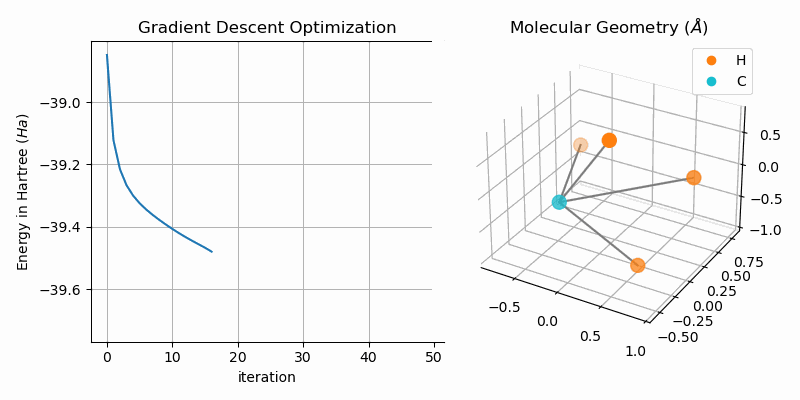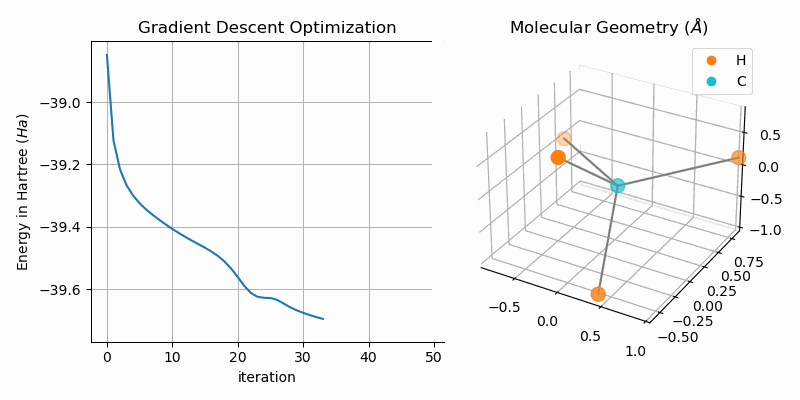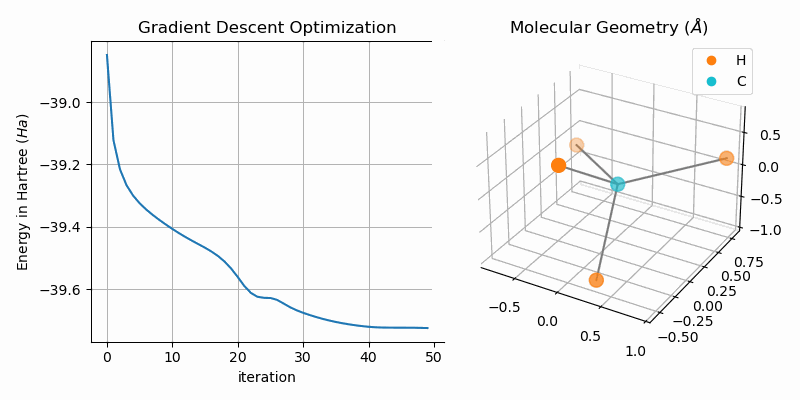
Molecular Geometry Optimization
The bond dissociation curves show a naive approach of finding stable states of bi-atomic molecules, i.e. low energy states. A more sophisticated approach is to directly minimize the energy of the system. One way of duing this is to apply gradient descent to the hartree fock energy. The GradientDescentGeometryOptimizer class implements exactly that. The following snipped shows the usage of the class (from examples/geometry.py):
optim = GradientDescentGeometryOptimizer(
mol=H1+H2+H3+H4+C,
alpha=0.03
)
# optimize molecular geometry
Es = optim.optimize(max_iters=100, tol=1e-5)
molecule = optim.moleculeAnimating the gradient descent optimization process for methane (
Future Work
- visualize bohmian trajectories for molecular orbits