This repository contains the conga python package and associated scripts
and workflows. conga was developed to detect correlation between
T cell gene expression profile and TCR sequence in single-cell datasets. We've just
recently added support for gamma delta TCRs and for B cells, too.
conga is in active development right now so the interface may change in
the next few months. Questions and requests can be directed to pbradley at fredhutch dot org.
Further details on conga can be found in the preprint entitled
"Linking T cell receptor sequence to transcriptional profiles with clonotype neighbor graph analysis (CoNGA)"
by Stefan A. Schattgen, Kate Guion, Jeremy Chase Crawford, Aisha Souquette, Alvaro Martinez Barrio, Michael J.T. Stubbington,
Paul G. Thomas, and Philip Bradley, accessible on the bioRxiv server
here.
Running conga on a single-cell dataset is a two- (or more) step process, as outlined below.
Python scripts are provided in the scripts/ directory but analysis steps can also be accessed interactively
in jupyter notebooks (for example, a simple pipeline and
Seurat to conga in the top directory of this repo)
or in your own python scripts through the interface in the conga python package.
The examples below and in the jupyter notebooks feature publicly available data from 10X Genomics,
which can be downloaded
using these links: GEX data and
TCR data.
- SETUP: The TCR data is converted to a form that can be read by
congaand then a matrix ofTCRdistdistances is computed. KernelPCA is applied to this distance matrix to generate a PC matrix that can be used in clustering and dimensionality reduction. This is accomplished with the python scriptscripts/setup_10x_for_conga.pyfor 10x datasets. For example:
python conga/scripts/setup_10x_for_conga.py --filtered_contig_annotations_csvfile vdj_v1_hs_pbmc_t_filtered_contig_annotations.csv --organism human
- ANALYZE: The
scripts/run_conga.pyscript has an implementation of the main pipeline and can be run as follows:
python conga/scripts/run_conga.py --graph_vs_graph --gex_data data/vdj_v1_hs_pbmc_5gex_filtered_gene_bc_matrices_h5.h5 --gex_data_type 10x_h5 --clones_file vdj_v1_hs_pbmc_t_filtered_contig_annotations_tcrdist_clones.tsv --organism human --outfile_prefix tmp_hs_pbmc
- RE-ANALYZE: Step 2 will generate a processed
.h5adfile that contains all the gene expression and TCR sequence information along with the results of clustering and dimensionality reduction. It can then be much faster to perform subsequent re-analysis or downstream analysis by "restarting" from those files.
python conga/scripts/run_conga.py --restart tmp_hs_pbmc_final.h5ad --graph_vs_tcr_features --graph_vs_gex_features --outfile_prefix tmp_hs_pbmc_restart
See the examples section below for more details.
conga relies heavily on the wonderful scanpy python package for single-cell analysis. See the scanpy
instructions for installation: https://scanpy.readthedocs.io/en/stable/installation.html.
We highly recommend using anaconda/miniconda for managing python environments. The calculations in the
conga manuscript were conducted with the following package versions:
scanpy==1.4.3 anndata==0.6.18 umap==0.3.9 numpy==1.16.2 scipy==1.2.1 pandas==0.24.1 scikit-learn==0.20.2 statsmodels==0.9.0 python-igraph==0.7.1 louvain==0.6.1
which might possibly be installed with the following conda command:
conda create -n conga_classic_env ipython python=3.6 scanpy=1.4.3 umap-learn=0.3.9
We've also been able to re-run everything, albeit with some numerical changes, with a current (2020-05-25) scanpy installation and these package versions:
scanpy==1.5.1 anndata==0.7.3 umap==0.4.3 numpy==1.17.5 scipy==1.4.1 pandas==1.0.3 scikit-learn==0.23.1 statsmodels==0.11.1 python-igraph==0.8.2 louvain==0.6.1 leidenalg==0.8.0
Which was installed with the following conda commands (following the scanpy docs):
conda create -n conga_new_env ipython python=3.6
conda activate conga_new_env (or source activate conga_new_env)
conda install seaborn scikit-learn statsmodels numba pytables
conda install -c conda-forge python-igraph leidenalg louvain
conda install -c intel tbb
pip install scanpy fastcluster
(And consider also adding conda install -c conda-forge notebook for Jupyter notebook stuff.)
Preliminary results suggest that, at least with default clustering parameters, the older louvain
clustering algorithm seems to give slightly 'better' results than the newer leiden algorithm,
ie finds a few more GEX/TCR associations, probably because there seem to be fewer, larger clusters.
If the louvain package is installed conga will use that.
Next, clone the conga repository:
git clone https://github.com/phbradley/conga.git
Then compile TCRdist using your C++ compiler.
We've successfullly used g++ from the GNU Compiler Collection (https://gcc.gnu.org/) to compile on
Linux and MacOS, and from MinGw (http://www.mingw.org/) for Windows.
Using make on Linux or MacOS
cd ~/conga/tcrdist_cpp
make
Or without make (for Windows)
cd ~/conga/tcrdist_cpp
g++ -O3 -std=c++11 -Wall -I ./include/ -o ./bin/find_neighbors ./src/find_neighbors.cc
g++ -O3 -std=c++11 -Wall -I ./include/ -o ./bin/calc_distributions ./src/calc_distributions.cc
We recommend using the write10XCounts function from the DropletUtils package for converting Seurat objects into 10x format for importing into CoNGA/scanpy.
require(Seurat)
require(DropletUtils)
hs1 <- readRDS('~/vdj_v1_hs_V1_sc_5gex.rds')
# write out for conga
write10xCounts(x = hs1@assays$RNA@counts, path = './hs1_mtx/')
# import the hs1_mtx directory into CoNGA using the '10x_mtx' option
-
2020-09-16: (EXPERIMENTAL) Added a preliminary implementation of the Hotspot autocorrelation algorithm developed by the Yosef lab, for finding informative features in multi-modal data (check out the github repo and the bioRxiv preprint). Hotspot finds numerical features whose pattern of variation across a single-cell dataset respects a user-supplied notion of cell-cell similarity (a neighbor graph with edge weights). We are using Hotspot to identify genes that respect the TCR neighbor graph, and TCR features that respect the gene expression neighbor graph (see examples in the Examples section below).
-
2020-09-16: Added a simple script for merging multiple datasets (
scripts/merge_samples.py). More functionality to come in the future; for the time being this will merge multiple datasets that each could be run through conga individually (ie clones files have already been generated with associated .barcode_mapping.tsv and kernel PCA files, and the barcodes in the barcode mapping files match those in the GEX data files. These conditions will be satisfied if each was generated byscripts/setup_10x_for_conga.py). The input to the script is a tab-separated values.tsvfile with three columns (corresponding to the three input arguments forscripts/run_conga.py:clones_filegex_dataandgex_data_type) which give the locations of the clonotype and gene expression data files. If these datasets are from the same individual and/or could contain cells from the same expanded clonotypes it might be worth using the arguments--condense_clonotypes_by_tcrdist --tcrdist_threshold_for_condensing 0.01which will merge clonotypes containing identical TCR sequences (for BCRs a larger tcrdist threshold value of 50ish might make sense). -
2020-09-04: (EXPERIMENTAL) Added support for bcrs and for gamma-delta TCRs. Right now
congauses the'organism'specifier to communicate the data type:humanandmousemean alpha-beta TCRs;human_gdandmouse_gdmean gamma-deltas;human_igmeans B cell data (not setup for mouse yet but let us know if that's of interest to you, for example by opening an Issue on github). Hacking the organism field like this allows us to put all the gene sequence information into a single, enlarged database file (conga/tcrdist/db/combo_xcrs.tsv). We still haven't updated all the image labels and filenames, so even though you are analyzing BCR data your plots will probably still say TCR in a few places...
The conga image-making pipeline requires an svg to png conversion. There seem to be a variety of
options for doing this, with the best choice being somewhat platform dependent. We've had good luck with
ImageMagick convert (on linux) and Inkscape (on mac). The conversion is handled in the file
conga/convert_svg_to_png.py, so you can modify that file if things are not working and you have
a tool installed; conga may not be looking in the right place. Also if the fonts
in the TCR/BCR logos look bad you could try switching the MONOSPACE_FONT_FAMILY
variable in that python file (see comments at the top of the file).
If you are having trouble and are using anaconda/miniconda, you could try
conda install -c conda-forge imagemagick in the relevant conda environment.
Shell scripts for running conga on three publicly available 10X
genomics datasets can be found in the examples/ directory:
examples/setup_all.bash which preprocesses the
clonotype data, and examples/run_all.bash which calls
scripts/run_conga.py on the three datasets. Here we discuss some of the
key outputs. For details on the algorithm and additional details on the plots,
refer to our bioRxiv preprint.
Here we focus on images, but the results of the different analysis
modes are also saved in a variety of tab-separated-values (*.tsv) files.
# DOWNLOAD FROM:
# https://support.10xgenomics.com/single-cell-vdj/datasets/3.1.0/vdj_v1_hs_pbmc3
# SETUP
/home/pbradley/anaconda2/envs/scanpy_new/bin/python /home/pbradley/gitrepos/conga/scripts/setup_10x_for_conga.py --organism human --filtered_contig_annotations_csvfile ./conga_example_datasets/vdj_v1_hs_pbmc3_t_filtered_contig_annotations.csv
# RUN
/home/pbradley/anaconda2/envs/scanpy_new/bin/python /home/pbradley/gitrepos/conga/scripts/run_conga.py --all --organism human --clones_file ./conga_example_datasets/vdj_v1_hs_pbmc3_t_filtered_contig_annotations_tcrdist_clones.tsv --gex_data ./conga_example_datasets/vdj_v1_hs_pbmc3_5gex_filtered_gene_bc_matrices_h5.h5 --gex_data_type 10x_h5 --outfile_prefix tcr_hs_pbmc
NOTE: In the RUN command above the --all argument to run_conga.py is a shorthand
for running all the current major modes of analysis, and is equivalent (right now)
to --graph_vs_graph --graph_vs_gex_features --graph_vs_tcr_features --cluster_vs_cluster --find_hotspot_features --find_gex_cluster_degs --make_tcrdist_trees.
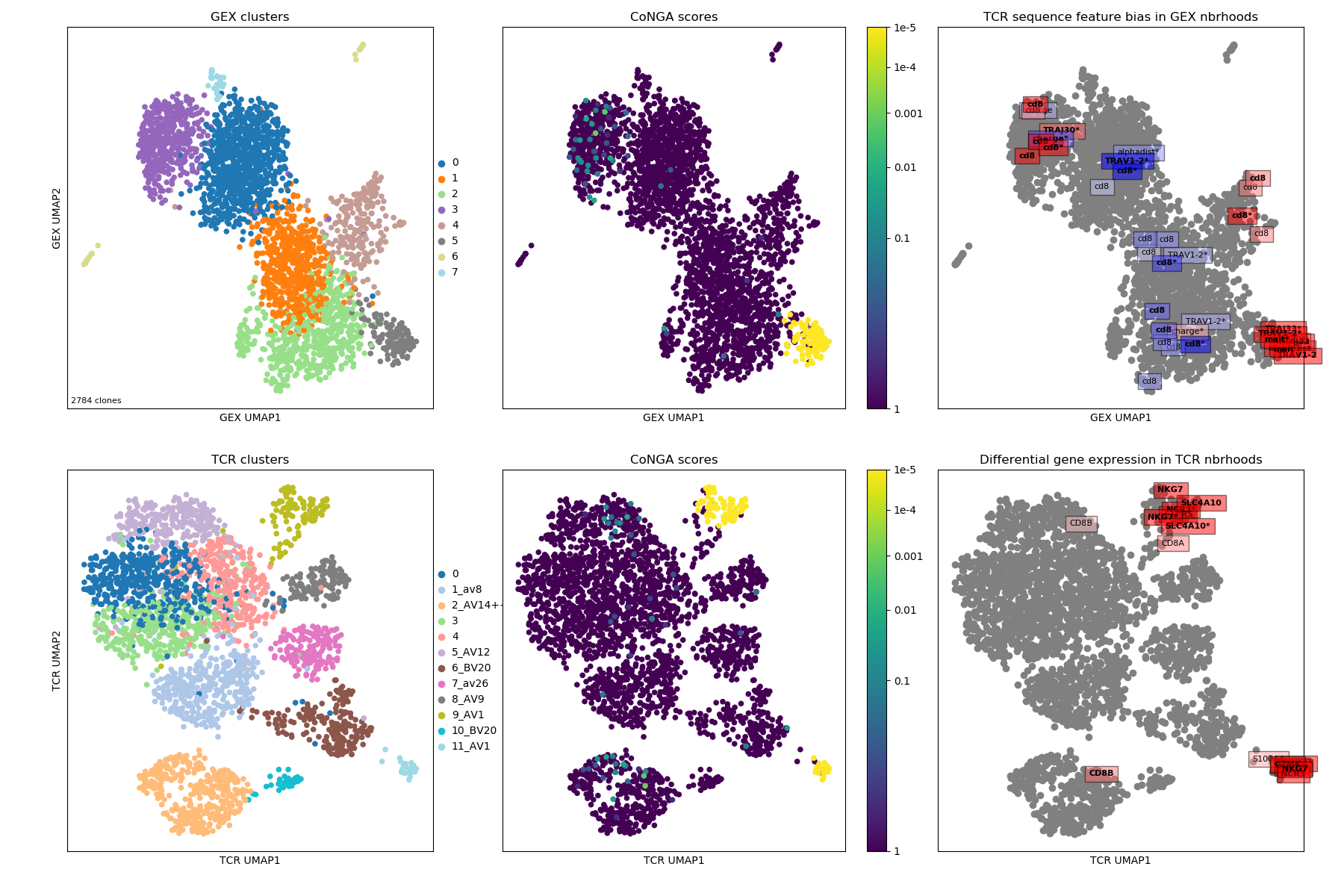
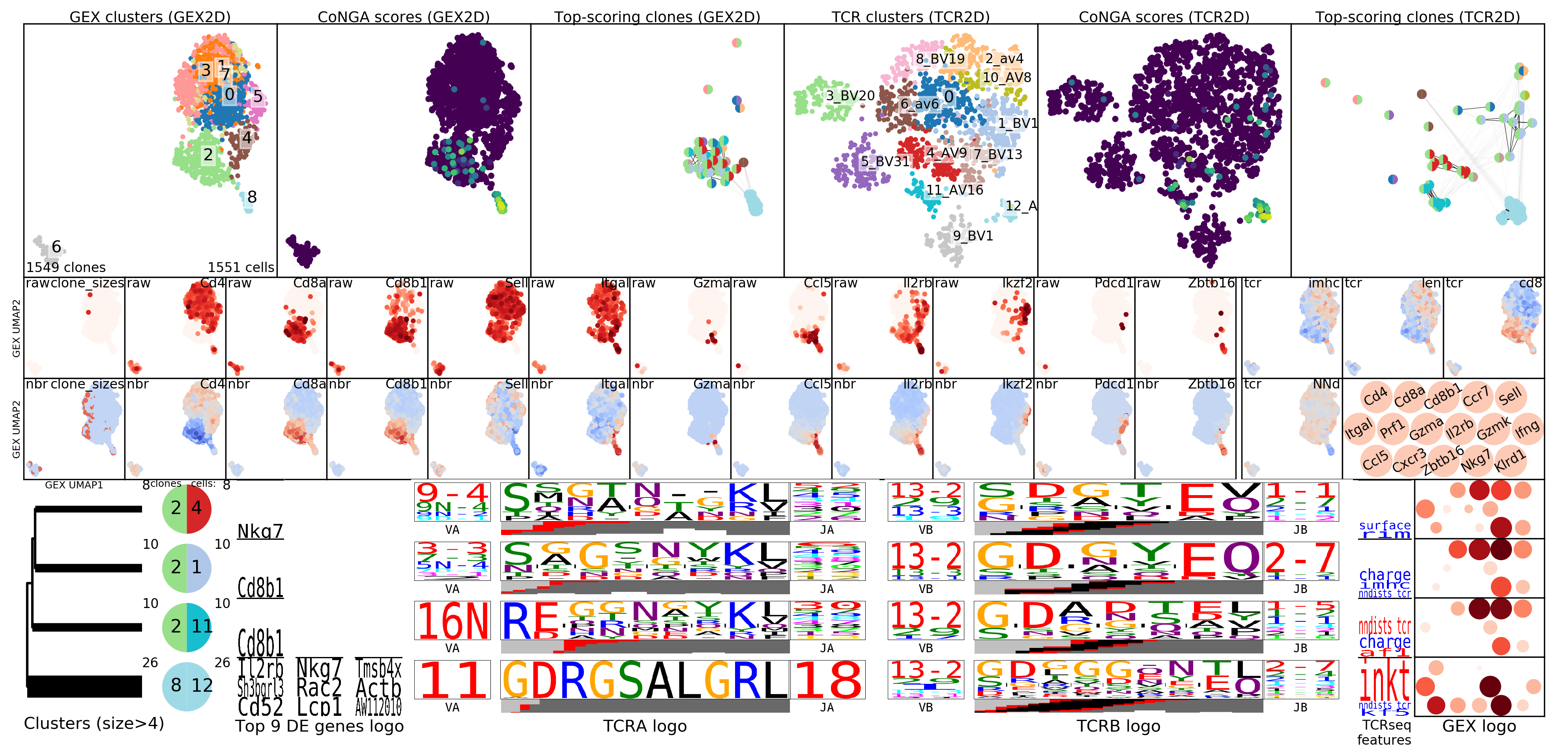
In looking at the CoNGA results a good place to start is with the summary image,
which will be named something
like OUTFILE_PREFIX_summary.png where OUTFILE_PREFIX was the --outfile_prefix
command line argument passed to run_conga.py. This image shows 2D projections of the
clonotypes in the dataset, based on distances in gene expression (GEX) space in
the top row and in TCR/BCR space in the bottom row. The left panels are colored
by clonotype clusters (GEX clusters on top and TCR clusters on the bottom);
the middle panels are colored by CoNGA score, which reflects overlap
between the GEX and TCR neighbor graphs on a per-clonotype basis; and the right
panels are overlaid with the top graph-vs-feature hits: TCR features that are
enriched in GEX graph neighborhoods on top, and genes that are differentially
expressed in TCR graph neighborhoods on the bottom.
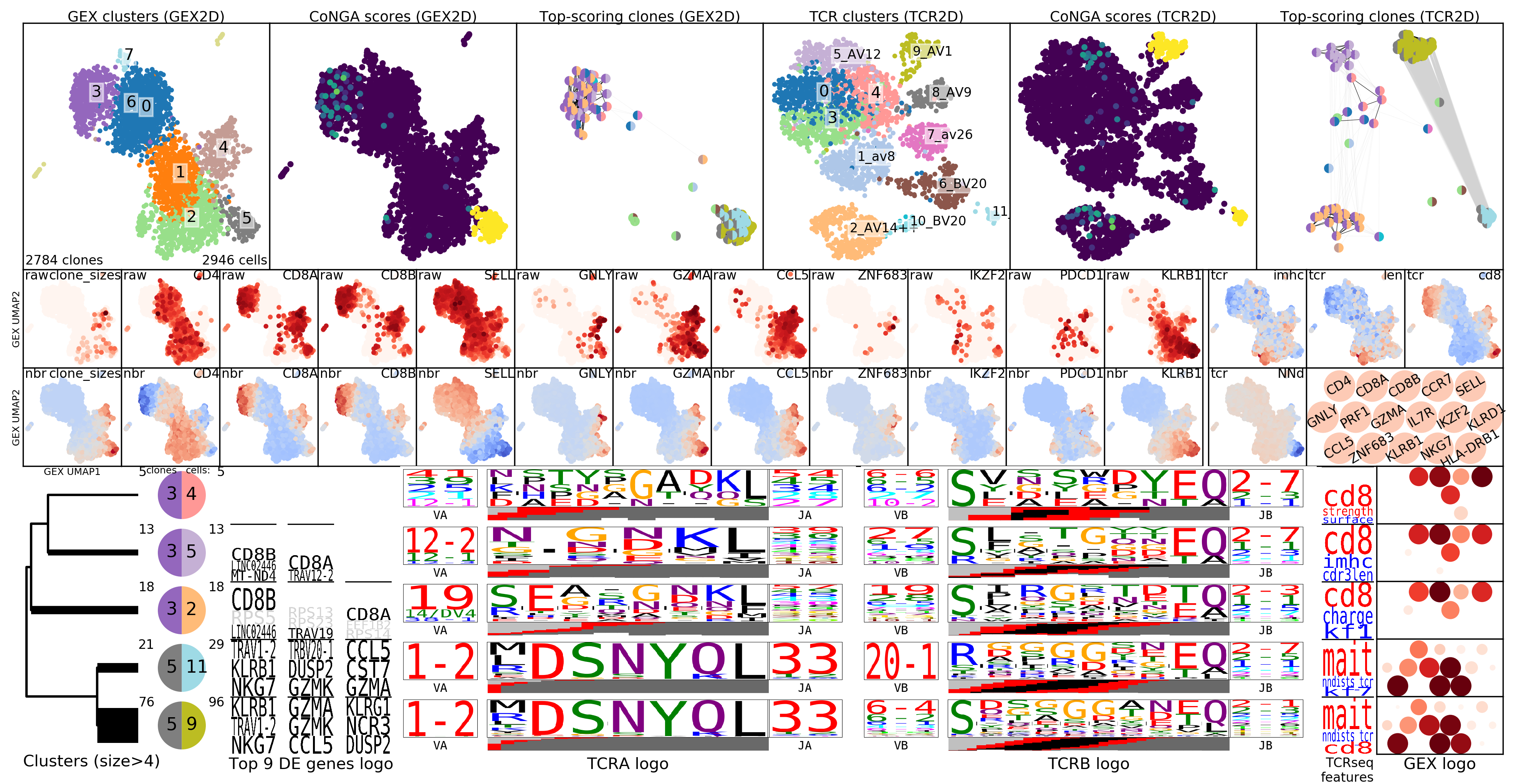
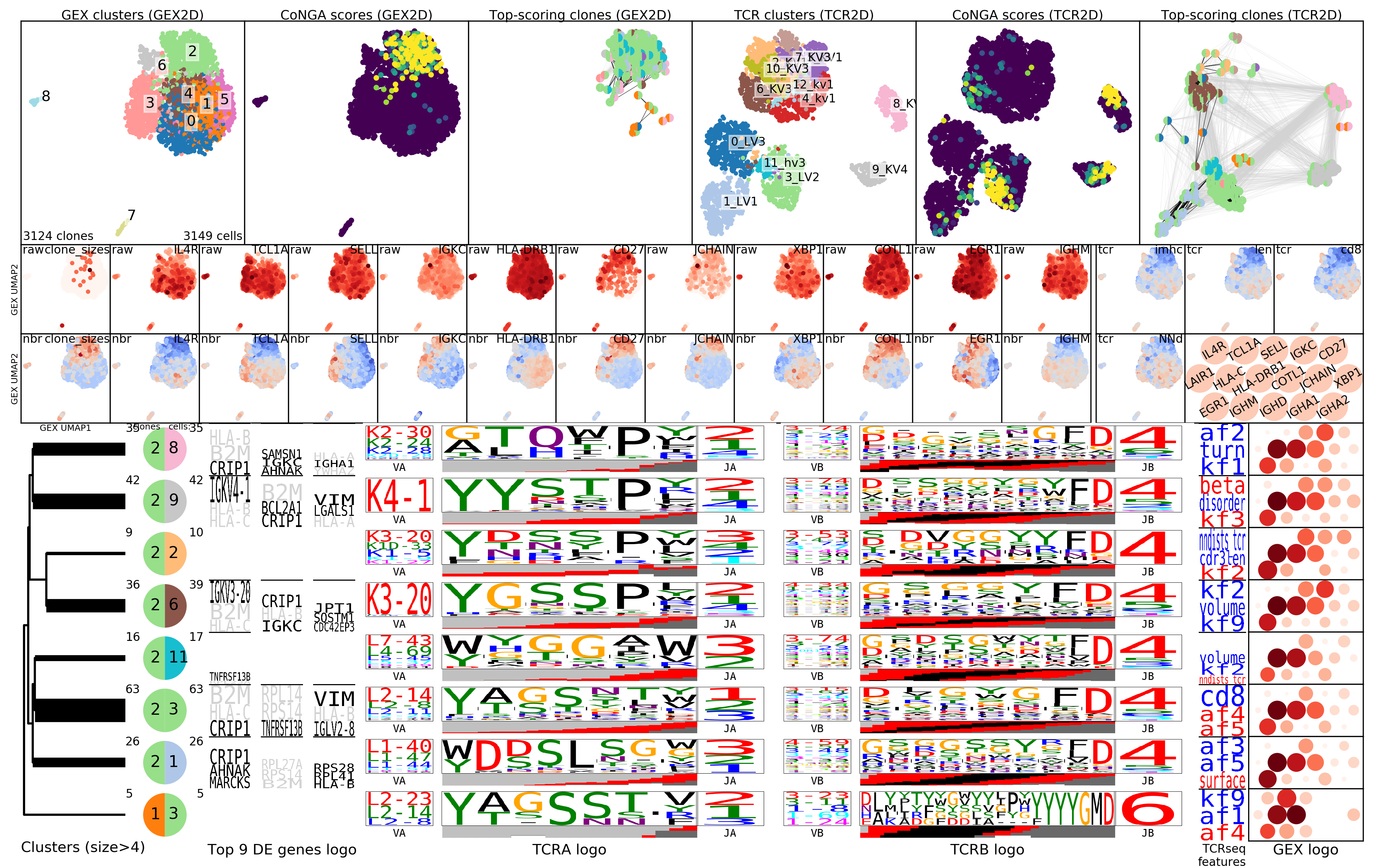
To gain greater insight into the clonotypes with significant CoNGA scores,
the software groups them by their joint GEX and TCR cluster assignments and
displays logos that summarize gene expression and TCR V/J gene usage and CDR3
sequence features for groups with size above a threshold (by default, at least
5 clonotypes and .1% of the overall dataset size). These logos are shown in the
plot OUTFILE_PREFIX_bicluster_logos.png. In this image the top panels are
show the clusters, conga scores, and conga hits (clonotypes with conga score<1)
in the GEX (left 3 panels) and TCR/BCR (right 3 panels) 2D lanscapes. Below
them are shown a sequence of thumbnail GEX projection scatter plots colored by
selected (and user configurable) marker genes; the top set are colored by raw
(normalized for cell reads and log+1 transformed) expression and in the bottom
set those values are Z-standardized and averaged over GEX graph neighborhoods,
to smooth and highlight overall trends. Below the snapshots are the rows of
cluster-pair (or 'bicluster') logos.
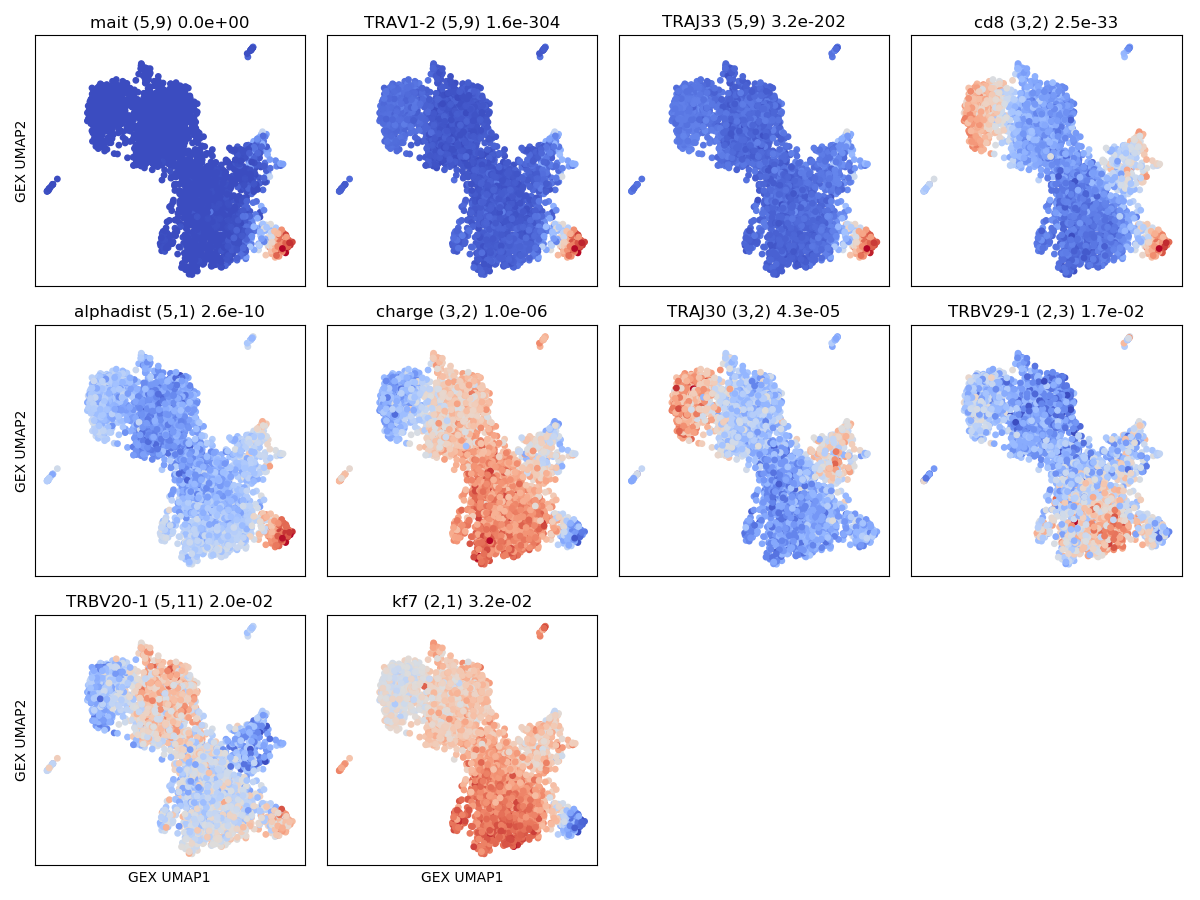
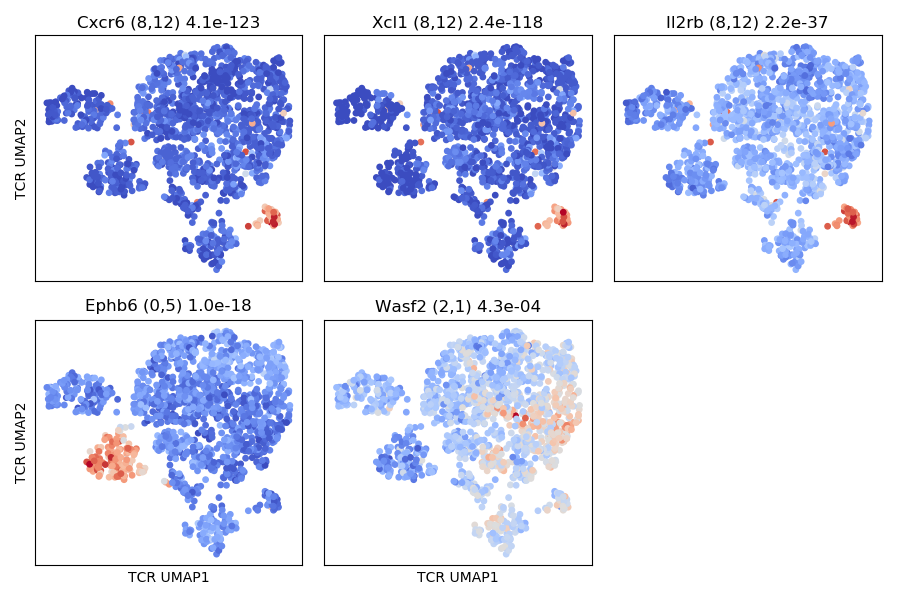
The results of the graph-vs-features analysis are summarized in a number of graphs
with names that include text like graph_vs_XXX_features. For example, the
top few TCR/GEX feature hits are shown projected onto the other landscape
(ie, TCR features onto the GEX landscape and GEX features onto the TCR landscape)
in plots that end in _panels.png, like the
OUTFILE_PREFIX_gex_nbr_graph_vs_tcr_features_panels.png shown below, where we can
see MAIT-cell features as well as features that differ between CD4 and CD8 T cells
(charge, TRBV20 and TRAJ30 usage). You can identify the CD4/CD8 cells by looking
at the CD4/CD8a/CD8b gene thumbnails in the cluster logos plot above.
In addition to the graph-vs-feature analysis described in the preprint,
we recently implemented a first, experimental version of the
HotSpot method
developed by the Yosef lab
(bioRxiv preprint).
Hotspot identifies numerical features that respect a given neighbor graph
structure, so we can use it to identify GEX features that correlate with
the TCR similarity graph and TCR/BCR features that correlate with the the
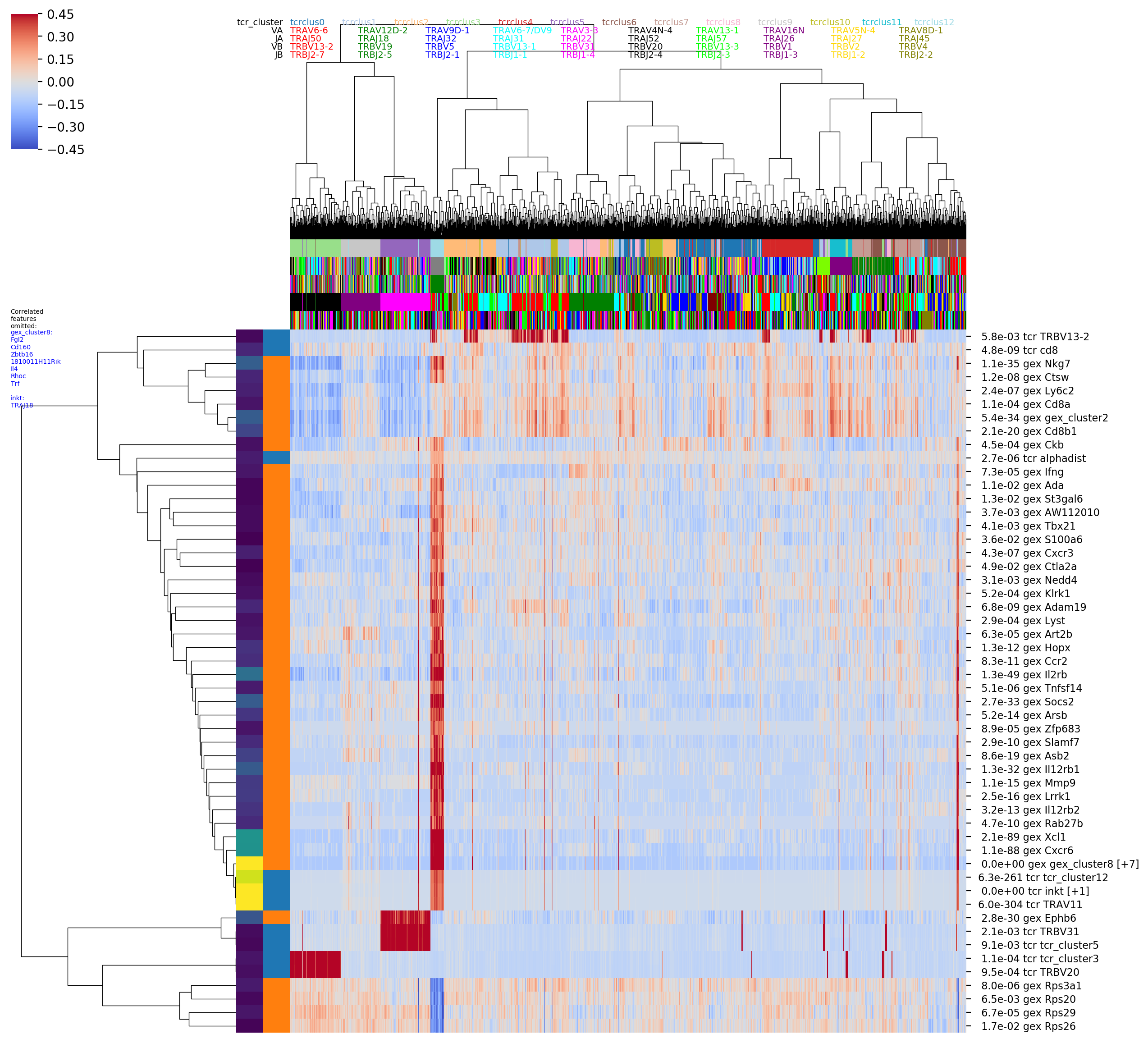
GEX similarity graph. These features are saved to tsv files and visualized
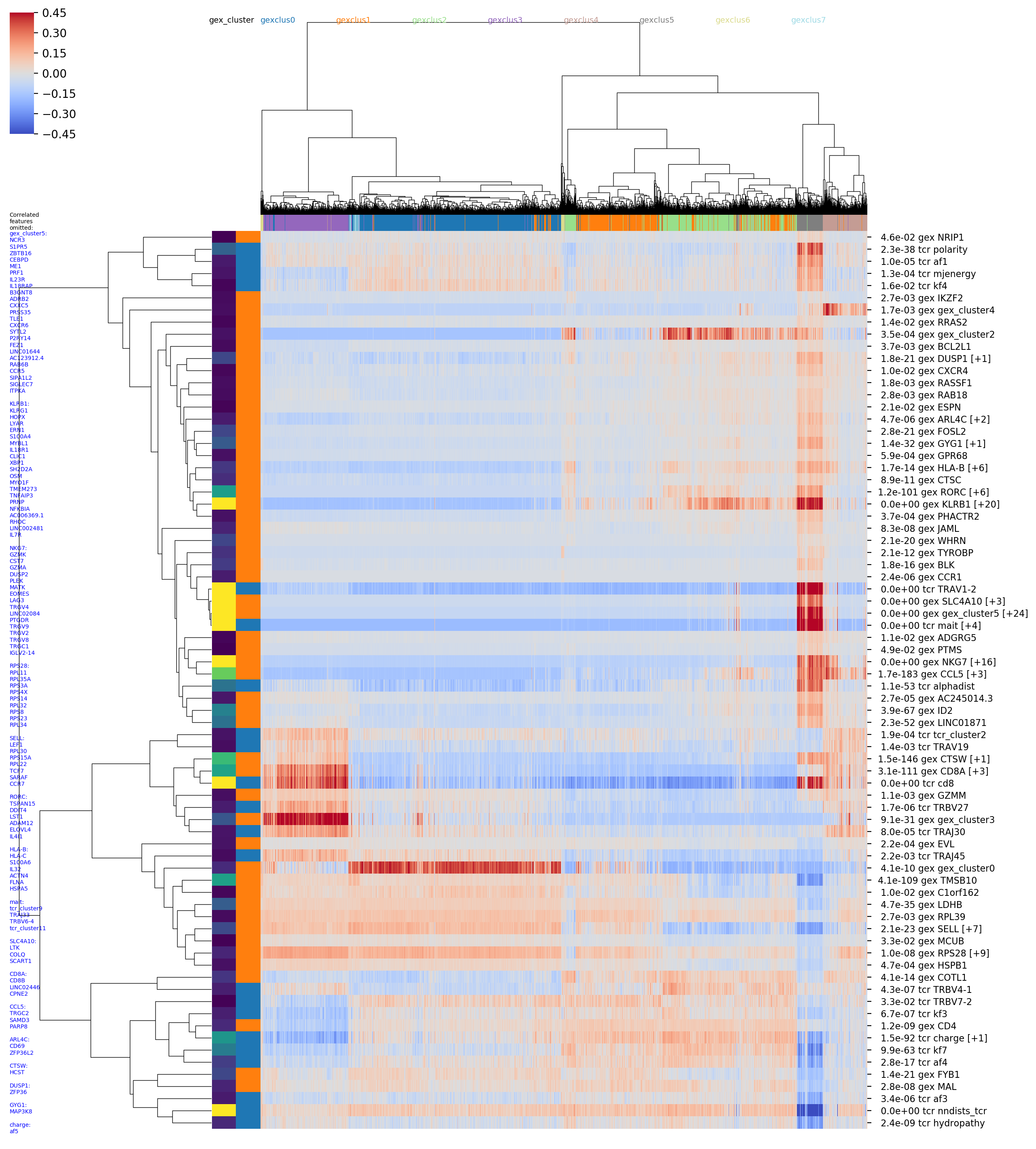
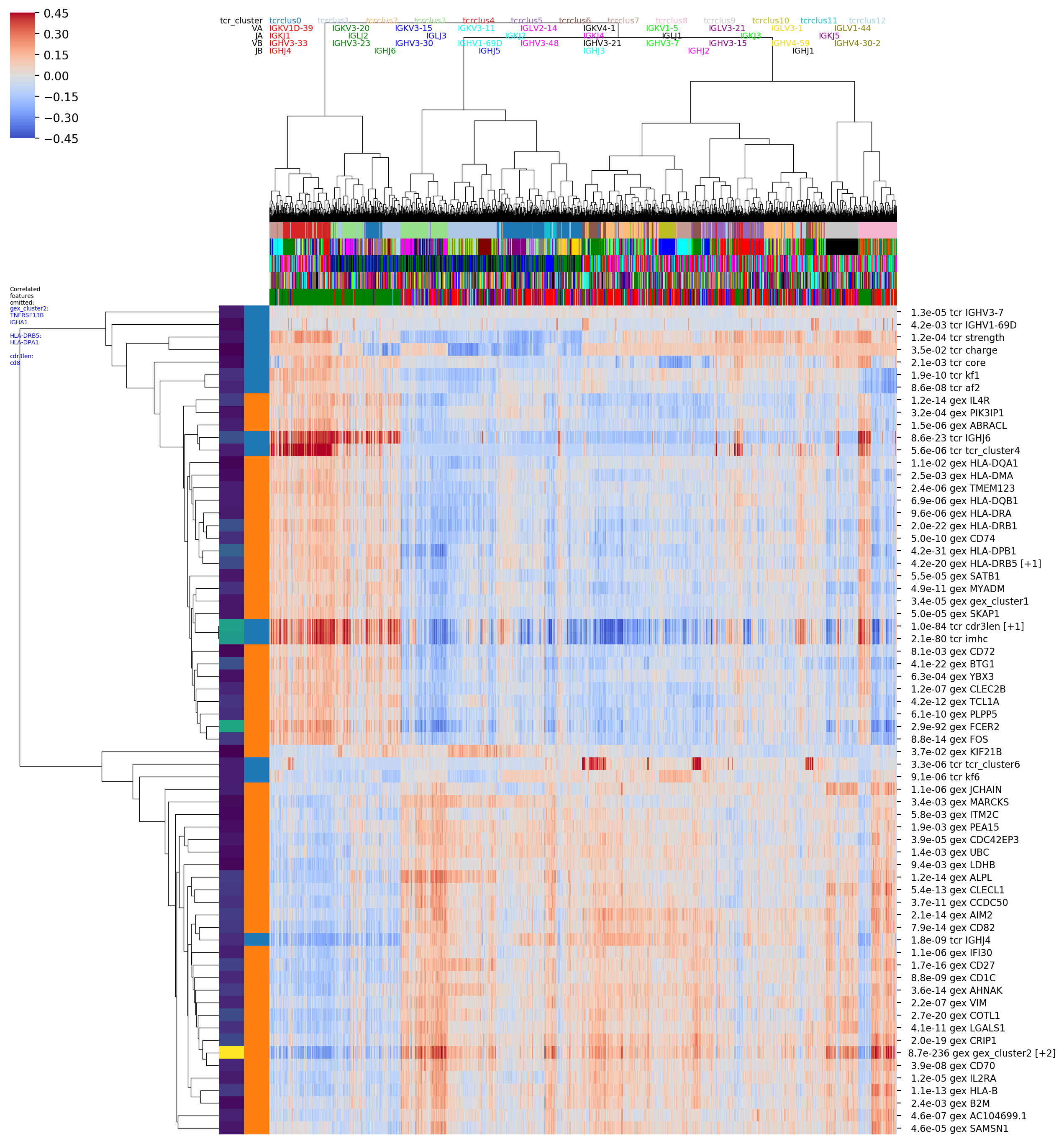
in a number of images, including seaborn clustermap plots if you have the
seaborn package installed. For example the image with the rather long
name
OUTFILE_PREFIX_0.100_nbrs_combo_hotspot_features_vs_gex_clustermap_lessredundant.png
shows the top combined GEX and TCR features (rows) versus clonotypes (columns), where the
clonotypes (columns) are ordered based on a hierarchical clustering dendrogram
built using GEX similarity (_vs_gex_clustermap in filename) and the scores
are averaged over GEX neighborhoods
(so that visible trends need to be consistent the GEX graph structure, and to smooth
noise). Features that are highly correlated with another, more significant
feature are filtered out and listed in the tiny blue text on the left (_lessredundant in
the filename). In this plot we can see MAIT, CD4, and CD8 groupings and many of the
same features as in the graph-vs-features analysis above. Since we have only
implemented the simplest model right now, the P-values may not be completely
trustworthy, and we suggest focusing on the results from larger neighbor
graphs (here we are showing the results for the graph with 0.1 neighbor fraction,
_0.100_nbrs in the filename,
ie where each clonotype is connected to the nearest 10 percent of the dataset).
The colors along the top of the matrix show the GEX cluster assignments of
each clonotype. The two columns of colors along the left-hand side of the matrix
show (left column) the P-value and (right column) the feature type (GEX vs TCR)
of the feature corresponding to that row (P-values and feature types are also
given in the row names along the right-hand side of the matrix). '[+N]' in the
row name means that N additional highly-correlated features were filtered out;
their names will be listed in the tiny blue text along the left-hand side of the
figure, listed below the name of the representative feature. (Some of these images
are big and very detailed-- downloading or opening in a separate tab and zooming
in may be helpful).
# DOWNLOAD FROM:
# https://support.10xgenomics.com/single-cell-vdj/datasets/3.0.0/vdj_v1_mm_balbc_pbmc_5gex
# SETUP
/home/pbradley/anaconda2/envs/scanpy_new/bin/python /home/pbradley/gitrepos/conga/scripts/setup_10x_for_conga.py --organism mouse --filtered_contig_annotations_csvfile ./conga_example_datasets/vdj_v1_mm_balbc_pbmc_t_filtered_contig_annotations.csv
# RUN
/home/pbradley/anaconda2/envs/scanpy_new/bin/python /home/pbradley/gitrepos/conga/scripts/run_conga.py --all --organism mouse --clones_file ./conga_example_datasets/vdj_v1_mm_balbc_pbmc_t_filtered_contig_annotations_tcrdist_clones.tsv --gex_data ./conga_example_datasets/vdj_v1_mm_balbc_pbmc_5gex_filtered_gene_bc_matrices_h5.h5 --gex_data_type 10x_h5 --outfile_prefix tcr_mm_pbmc
The summary image, where we can see a tight cluster of conga hits that turn out
to be iNKT cells (inkt TCR feature enriched in top right panel), some CD8-enriched
genes (TRAV16N and TRBV29), and the EphB6 gene which turns out to be correlated
with usage of the TRBV31 gene:
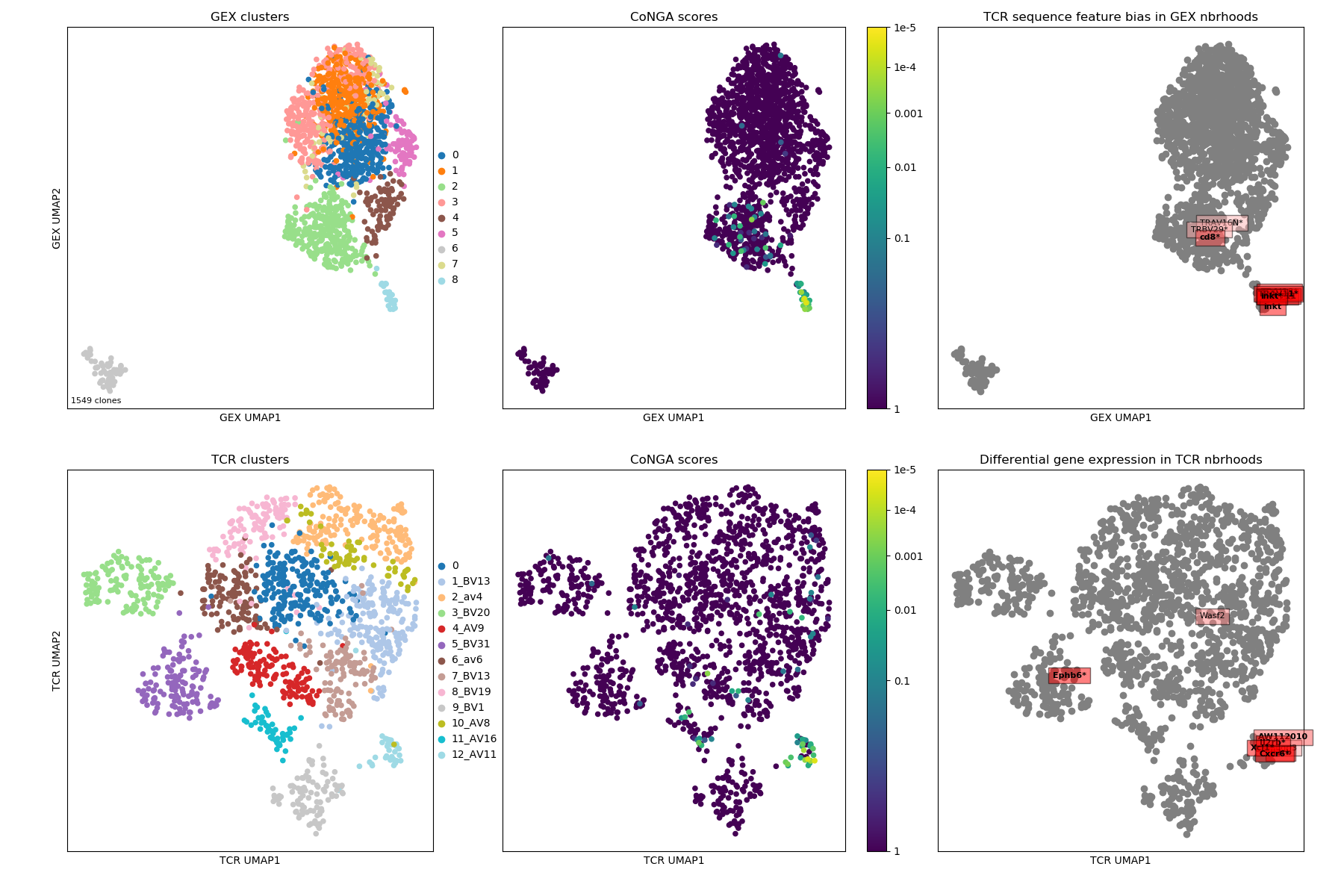
Here the cluster logo image shows iNKT cells and some CD8-positive clusters that likely reflect TCR sequence features shared by CD8s.
The top few GEX features that are enriched in TCR neighborhoods: some iNKT genes and the EphB6 gene showing localized expression in the TCR landscape projection (in the TRBV31 expressing clonotypes).
In the hotspot clustermap showing features versus TCR-arranged clonotypes we can see the correlation between EphB6 and TRBV31 nicely. The color rows along the top of the matrix show (from top to bottom) the TCR cluster assignment and the TRAV, TRAJ, TRBV, and TRBJ gene segment usage patterns (color key for the top few genes is shown at the top of the column dendrogram).
# DOWNLOAD FROM:
# https://support.10xgenomics.com/single-cell-vdj/datasets/4.0.0/sc5p_v1p1_hs_melanoma_10k
# SETUP
/home/pbradley/anaconda2/envs/scanpy_new/bin/python /home/pbradley/gitrepos/conga/scripts/setup_10x_for_conga.py --organism human_ig --filtered_contig_annotations_csvfile ./conga_example_datasets/sc5p_v1p1_hs_melanoma_10k_b_filtered_contig_annotations.csv --condense_clonotypes_by_tcrdist --tcrdist_threshold_for_condensing 50
# RUN
/home/pbradley/anaconda2/envs/scanpy_new/bin/python /home/pbradley/gitrepos/conga/scripts/run_conga.py --all --organism human_ig --clones_file ./conga_example_datasets/sc5p_v1p1_hs_melanoma_10k_b_filtered_contig_annotations_tcrdist_clones_condensed.tsv --gex_data ./conga_example_datasets/sc5p_v1p1_hs_melanoma_10k_filtered_feature_bc_matrix.h5 --gex_data_type 10x_h5 --outfile_prefix bcr_hs_melanoma
Two features to note in the commands above: (1) we are passing --organism human_ig
to let conga know we are working with BCR data, (2) in the setup command we
added the flags --condense_clonotypes_by_tcrdist --tcrdist_threshold_for_condensing 50,
which trigger merging of 10X clonotypes whose TCRdist (actually BCR dist) is
less than or equal to 50 (ie, we do single-linkage clustering and cut the tree
at a distance threshold of 50). Here the goal is to merge clonally related
families so that GEX/BCR covariation that we detect reflects the correlation
across independent rearrangements. The user could instead apply a more
sophisticated clone identification procedure and modify the raw_clonotype_id
column in the contigs csv file, which is where conga gets the clonotype info.
The summary image, where we can see that most of the conga hits are in GEX cluster
2; that there are differences in CDR3 length across the landscape; and some
specific genes that are differentially expressed in TCR graph neighborhoods
(TNFRSF13B, B2M, CRIP1).
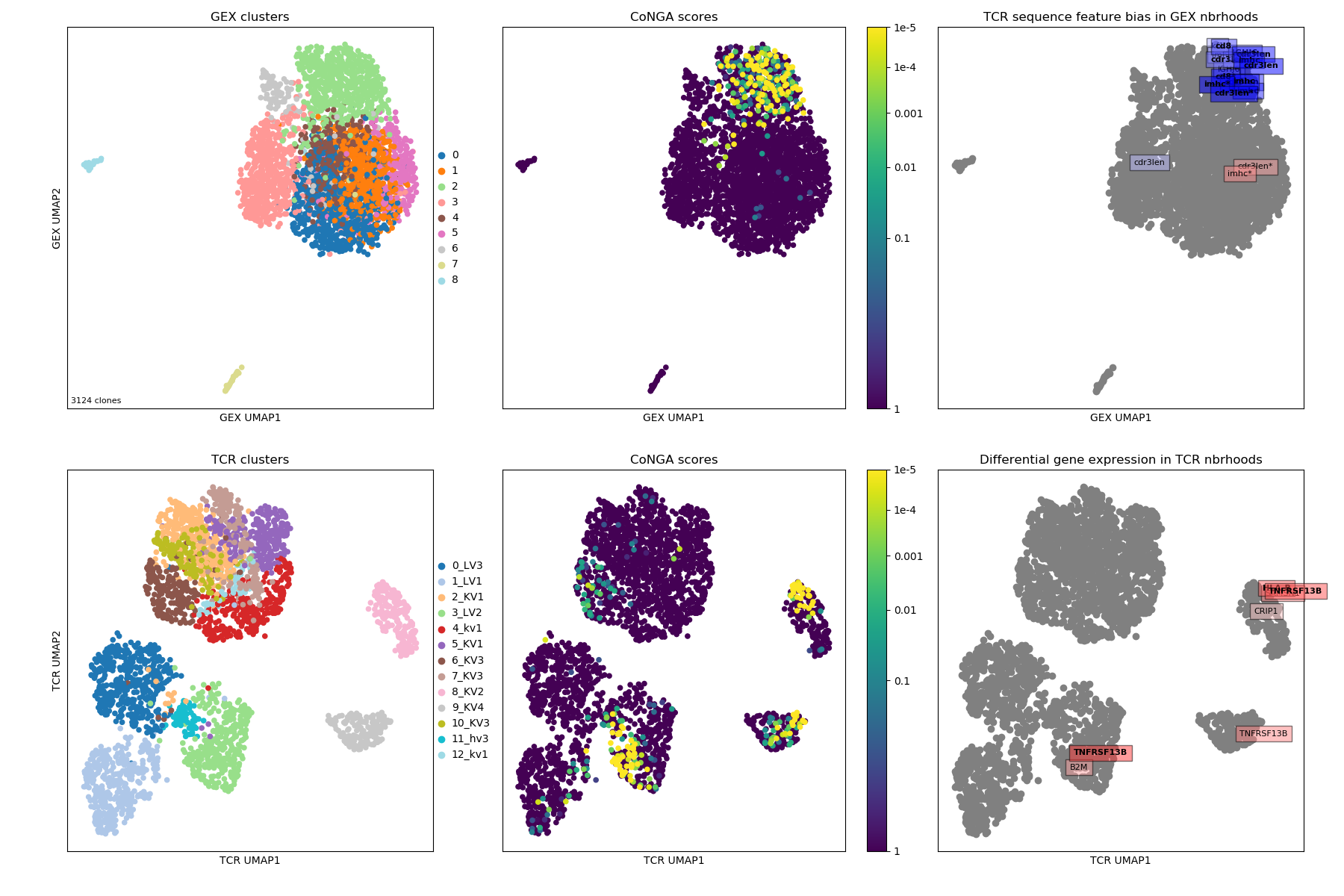
Logos for the clusters of conga hits, with one cluster of 'naive' clonotypes with long CDRH3s and high TCL1A expression.
In the hotspot clustermap showing features versus TCR-arranged clonotypes we can see a breakdown between naive and non-naive features, where IGHJ6 and CDR3 length are correlated with genes like TCL1A and class II HLA genes, and IGHJ4 is enriched in the non-naives.
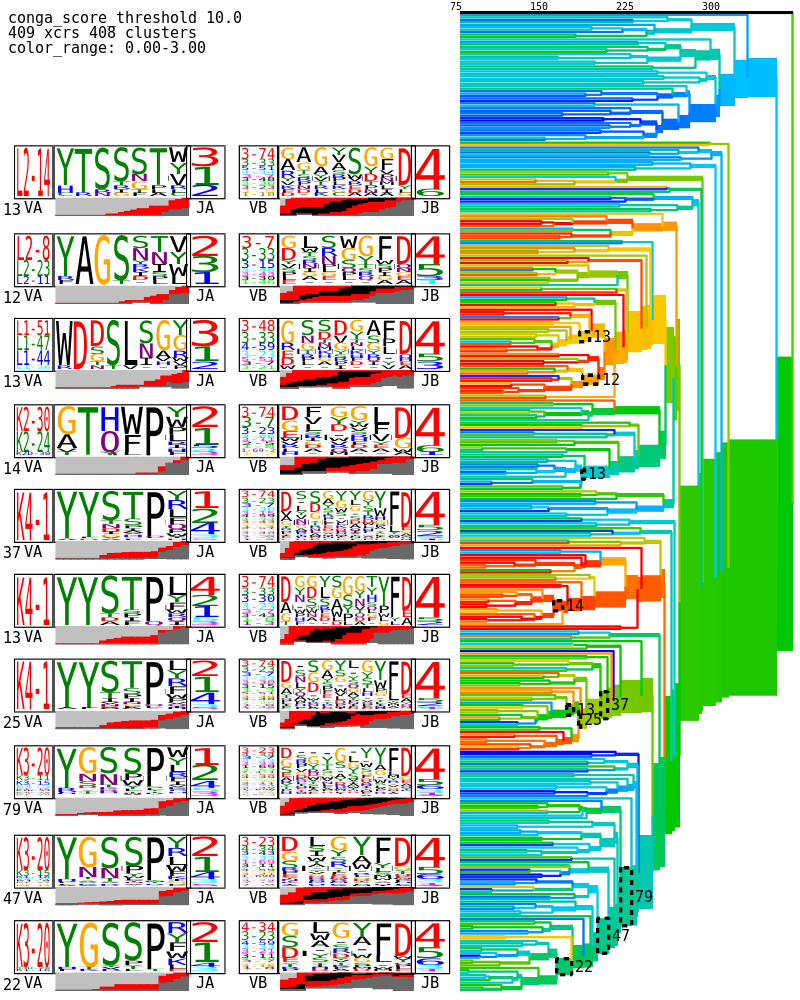
CoNGA also makes a tree of all the clonotypes with a conga score less than a loose threshold value of 10, where we can look for sequence clustering. The branches are colored by a transformed version of the conga score that maps the threshold value of 10 to zero (blue) with more significant scores trending toward red at a value of 1e-8: