OrthoFinder — Accurate inference of orthologs, orthogroups, the rooted species, gene trees and gene duplcation events tree made easy!
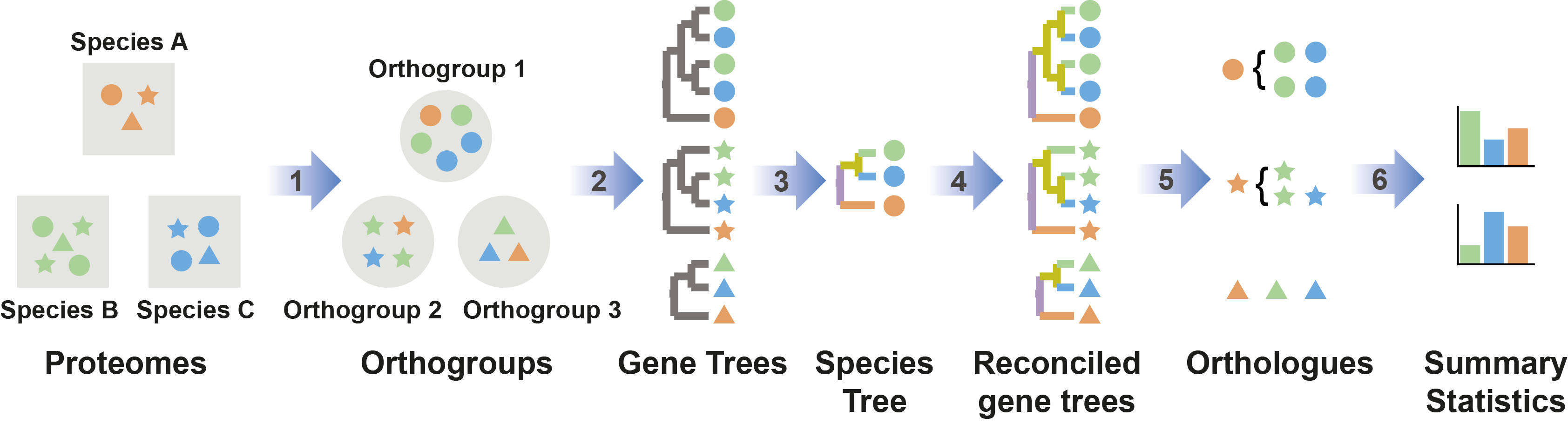 Figure 1: Automatic OrthoFinder analysis
Figure 1: Automatic OrthoFinder analysis
OrthoFinder is a fast, accurate and comprehensive platform for comparative genomics. It finds orthologs and orthogroups, infers rooted gene trees for all orthogroups and identifies all of the gene duplcation events in those gene trees. It also infers a rooted species tree for the species being analysed and maps the gene duplication events from the gene trees to branches in the species tree. OrthoFinder also provides comprehensive statistics for comparative genomic analyses. OrthoFinder is simple to use and all you need to run it is a set of protein sequence files (one per species) in FASTA format.
For more details see the OrthoFinder paper below.
Emms, D.M. and Kelly, S. (2015) OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biology 16:157
https://genomebiology.biomedcentral.com/articles/10.1186/s13059-015-0721-2
- What are orthogroups, orthologs & paralogs?
- Why use orthogroups in your analysis
- Installing OrthoFinder
- Running OrthoFinder
- OrthoFinder results files and statistics
- Adding and removing species from a completed OrthoFinder run
- Preparing and using seperately run BLAST files
Orthologs are pairs of genes that descended from a single gene in the last common ancestor (LCA) of two species (Figure 2A & B). An orthogroup is the extension of the concept of orthology to groups of species. An orthogroup is the group of genes descended from a single gene in the LCA of a group of species (Figure 2A).
The example Figure 2 contains an orthogroup from three species, human, mouse and chicken. Human and mouse each have one gene in this orthogroup (HuA and MoA, respectively) while chicken has two genes (ChA1 and ChA2). The human and mouse genes are a pair of genes descended from a single gene in the last common ancestor of the two species, therefore these two genes are orthologs and there is a one-to-one orthology relationship between the two genes.
The two chicken genes arose from a gene duplication event after the lineage leading to chicken split from the lineage leading to human and mouse. As gene duplication events give rise to paralogs, ChA1 and ChA2 are paralogs of each other. However, both chicken genes are decended from a single gene in the last common ancestor of the three species. Therefore both chicken genes are orthologs of the human gene and the mouse gene. Although they are orthologs, sometimes these complex relationships are reffered to as co-orthologs (e.g. ChA1 and ChA2 are co-orthologs of HuA). In this case there is a many-to-one orthology relationship between the chicken genes and the human gene. There are only three kinds of orthology relationships one-to-one, many-to-one, and many-to-many. All of these relationships are identified by OrthoFinder.
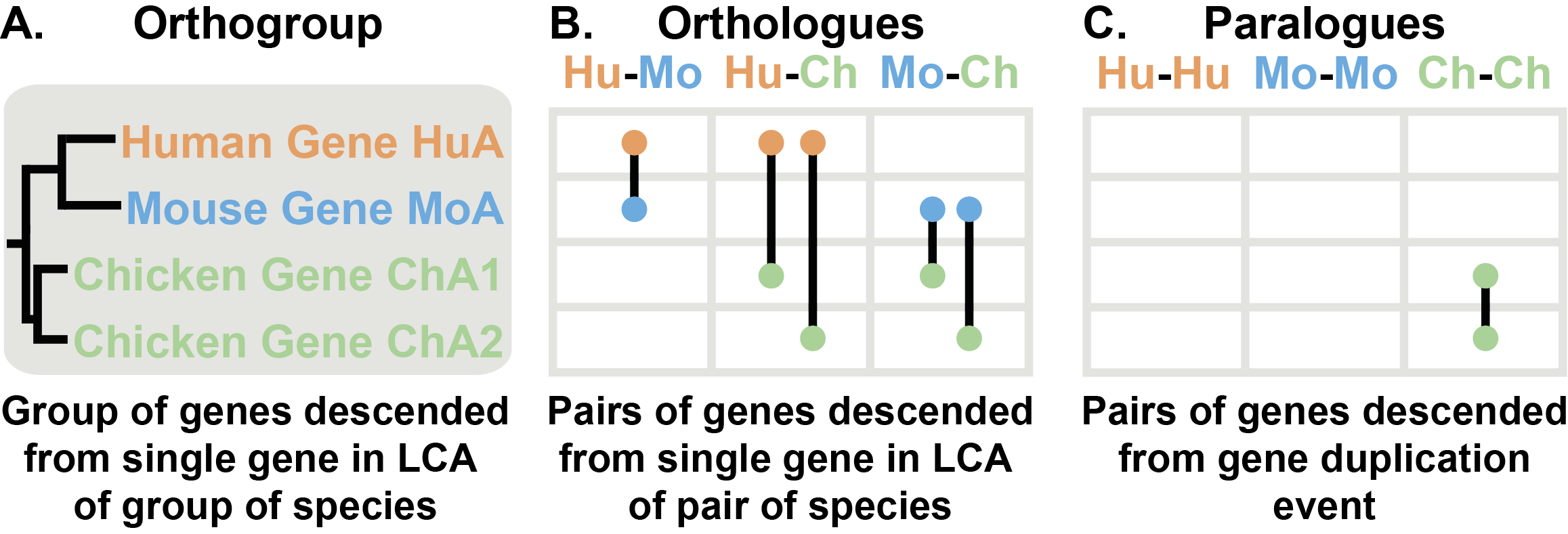 Figure 2: Orthologues, Orthogroups & Paralogues
Figure 2: Orthologues, Orthogroups & Paralogues
All of the genes in an orthogroup are decended from a single ancestral gene. Thus all the genes in an orthogroup started out with the same sequence and function. As gene duplication and loss occur frequently in evolution, one-to-one orthologs are rare and limitation of analyses to on-to-one othologs limits an analysis to a small fraction of the available data. By analysing orhtogroups you can analyse all of your data.
It is important to note that with orthogroups you choose where to define the limits of the unit of comparison. For example, if you just chose to analyse human and mouse in the above figure then you would have two orthogoups.
Orthology is defined by phylogeny. It is not definable by amino acid content, codon bias, GC content or other measures of sequence similarity. Methods that use such scores to define orthologs in the absence of phylogeny can only provide guesses. To provide a crude analogy guessing orthology from sequence similarity is akin to guessing colour from smell. The only way to truly identify orthologs is thus through analysis of phylogenetic trees. The only way to be sure that the orthology assignment is correct is by conducting a phylogenetic reconstruction of all genes decended from a single gene the last common ancestor of the species under consideration. This set of genes is an orthogroup. Thus the only way to define orthology is by analysing orthogroups.
OrthoFinder runs on Linux and Mac, setup instructions are given below.
-
Download the latest release from github: https://github.com/davidemms/OrthoFinder/releases (for this example we will assume it is OrthoFinder-2.2.7.tar.gz, change this as appropriate.)
-
In a terminal, 'cd' to where you downloaded the package
-
Extract the files:
tar xzf OrthoFinder-2.2.7.tar.gz -
Test you can run OrthoFinder:
OrthoFinder-2.2.7/orthofinder -h. OrthoFinder should print its 'help' text.
To perform an analysis OrthoFinder requires some dependencies to be installed and in the system path (only the first two are needed to infer orthogroups and all four are needed to infer orthologues and gene trees as well):
-
DIAMOND or MMseqs2 (recommended, although BLAST+ can be used instead)
-
The MCL graph clustering algorithm
-
FastME (The appropriate version for your system, e.g. 'fastme-2.1.5-linux64', should be renamed `fastme', see instructions below.)
Brief instructions are given below although users can refer to the installation notes provided with these packages for more detailed instructions.
Each of the following packages provide their own detailed instructions for installation, here we give a concise guide.
Available here: https://github.com/bbuchfink/diamond/releases
Download the the latest release, extract it and copy the executable to a directory in your system path, e.g.:
wget https://github.com/bbuchfink/diamond/releases/download/v0.9.22/diamond-linux64.tar.gztar xzf diamond-linux64.tar.gzsudo cp diamond /usr/local/bin
or alternaitvely if you don't have root privileges, instead of the last step above, add the directory containing the directory to your PATH variable. E.g
mkdir ~/bincp diamond ~/binexport PATH=$PATH:~/bin/
Available here: https://github.com/soedinglab/MMseqs2/releases
Download the appropriate version for your machine, extract it and copy the executable to a directory in your system path, e.g.:
wget https://github.com/soedinglab/MMseqs2/releases/download/3-be8f6/MMseqs2-Linux-AVX2.tar.gztar xzf MMseqs2-Linux-AVX2.tar.gzsudo cp mmseqs2/bin/mmseqs /usr/local/bin
or alternaitvely if you don't have root privileges, isntead of the last step above, add the directory containing the directory to your PATH variable
export PATH=$PATH:`pwd`/mmseqs2/bin/
The mcl clustering algorithm is available in the repositories of some Linux distributions and so can be installed in the same way as any other package. For example, on Ubuntu, Debian, Linux Mint:
sudo apt-get install mcl
Alternatively it can be built from source which will likely require the 'build-essential' or equivalent package on the Linux distribution being used. Instructions are provided on the MCL webpage, http://micans.org/mcl/.
FastME can be obtained from http://www.atgc-montpellier.fr/fastme/binaries.php. The package contains a 'binaries/' directory. Choose the appropriate one for your system and copy it to somewhere in the system path e.g. '/usr/local/bin'** and name it 'fastme'. I.e.:
sudo cp fastme-2.1.5-linux64 /usr/local/bin/fastme
NCBI BLAST+ is available in the repositories from most Linux distributions and so can be installed in the same way as any other package. For example, on Ubuntu, Debian, Linux Mint:
sudo apt-get install ncbi-blast+
Alternatively, instructions are provided for installing BLAST+ on Mac and various flavours of Linux on the "Standalone BLAST Setup for Unix" page of the BLAST+ Help manual currently at http://www.ncbi.nlm.nih.gov/books/NBK1762/. Follow the instructions under "Configuration" in the BLAST+ help manual to add BLAST+ to the PATH environment variable.
Once the required dependencies have been installed, try running OrthoFinder on the example data:
OrthoFinder-1.0.6/orthofinder -f ExampleDataset -S diamond
Assuming everything was successful OrthoFinder will end by printing the location of the results files, a short paragraph providing a statistical summary and the OrthoFinder citation. If you make use of OrthoFinder for any of your work then please cite it as this helps support future development.
If you have problems with this standalone binary version of OrthoFinder you can use the python source code version, which has a name of the form, 'OrthoFinder-1.0.6_source.tar.gz' and is available from the github 'releases tab'. See Section 'Python Source Code Version'.
The following steps are not required for the standard OrthoFinder use cases and are only needed if you want to infer maximum likelihood trees from multiple sequence alignments (MSA). This is considerably more costly computationally but more accurate. By default MAFFT is used for the alignment and FastTree for the tree inference. Both the executables should be in the system path. The option for this is, "-M msa".
You can actually use any alignment or tree inference program you like the best! Be careful with the method you chose, OrthoFidner typically needs to infer about 10,000-20,000 gene trees. If you have many species or if the tree/alignment method isn't super-fast then this can take a very long time! MAFFT + FastTree provides a reasonable compromise. Orthofinder already knows how to call:
- mafft
- muscle
- iqtree
- raxml
- raxml-ng
- fasttree
If you want to use a different program, there is a simple configuration file called "config.json" in the orthofinder directory. You just need to add an entry to tell it what the command line looks like for the program you want to use. There are lots of examples in the file that you can follow.
For example, to you muscle and iqtree, the command like arguments you need to add are: "-M msa -A muscle -T iqtree"
It is recommended that you use the standalone binaries for OrthoFinder which do not require python or scipy to be installed. However, the python source code version is available from the github 'releases' page (e.g. 'OrthoFinder-1.0.6_source.tar.gz' and requires python 2.7 and scipy to be installed. Up-to-date and clear instructions are provided here: http://www.scipy.org/install.html, be sure to chose a version using python 2.7. As websites can change, an alternative is to search online for "install scipy".
Performing a complete OrthoFinder analysis is simple:
- Download the amino acid sequences, in FASTA format, for the species you want to analyse. If you have the option, it is best to use a version containing a single representative/longest transcript-variant for each gene.
- Optionally, you may want to rename the files to something simple since the filenames will be used as species identifiers in the results. E.g if you were using the 'Homo_sapiens.GRCh38.pep.all.fa' file you could rename it to 'Homo_sapiens.fa' or 'Human.fa'.
- Place the FASTA files all in a single directory.
- To perform a complete OrthoFinder analysis requires just one command:
orthofinder -f fasta_files_directory [-t number_of_threads]
The argument 'number_of_threads' is an optional argument to specify the number of parallel threads to use for the BLAST searches, tree inference and reconciliation. As the BLAST queries can be a time-consuming step it is best to use at least as many BLAST processes as there are CPUs on the machine.
The OrthoFinder run will finish by printing the location of the results files, a short paragraph providing a descriptive statistical summary and the OrthoFinder citation. If you make use of OrthoFinder for any of your work then please cite it as this helps justify OrthoFinder support and future development. The OrthoFinder results files are described in the section "Results Files".
A standard OrthoFinder run produces a set of files describing the orthogroups, orthologues and gene trees for the set of species being analysed. Their locations are given at the end of an OrthoFinder run.
OrthoFinder generates the main orthogroup file, 'Orthogroups.csv', and two supporting files:
-
Orthogroups.csv is a tab separated text file. Each row contains the genes belonging to a single orthogroup. The genes from each orthogroup are organized into columns, one per species.
-
Orthogroups_UnassignedGenes.csv is a tab separated text file that is identical in format to Orthogroups.csv but contains all of the genes that were not assigned to any orthogroup.
-
Orthogroups.txt (legacy format) is a second file containing the orthogroups described in the Orthogroups.csv file but using the OrthoMCL output format.
The statistics calculated from the orthogroup analysis provide the basis for any comparative genomics analysis. They are easily plotted and can also be used for quality control.
-
Statistics_Overall.csv is a tab separated text file giving useful statistics from the orthogroup analysis.
-
Statistics_PerSpecies.csv is a tab separated text file giving many of the same statistics as the 'Statistics_Overall.csv' file but on a species-by-species basis.
-
Orthogroups_SpeciesOverlaps.csv is a tab separated text file containing a matrix of the number of orthogroups shared by each species-pair (i.e. the number of orthogroups which contain at least one gene from each of the species-pairs).
Most of the terms in the files 'Statistics_Overall.csv' and 'Statistics_PerSpecies.csv' are self-explanatory, the remainder are defined below.
- Species-specific orthogroup: An orthogroups that consist entirely of genes from one species.
- G50: The number of genes in the orthogroup such that 50% of genes are in orthogroups of that size or larger.
- O50: The smallest number of orthogroups such that 50% of genes are in orthogroups of that size or larger.
- Single-copy orthogroup: An orthogroup with exactly one gene (and no more) from each species. These orthogroups are ideal for inferring a species tree and many other analyses.
- Unassigned gene: A gene that has not been put into an orthogroup with any other genes.
The orthologues spreadsheets are contained in sub-directories, one per species. Within these directories is one spreadsheet per species-pair giving all the inferred orthologues between those two species. The spreadsheets contain one column for the genes from one species and one column for genes from the other species. Orthologues can be one-to-one, one-to-many or many-to-many depending on the gene duplication events since the orthologues diverged (see Section "Orthogroups, Orthologues & Paralogues" for more details). Each set of orthologues is cross-referenced to the orthogroup that contains them.
The gene trees for each orthogroup and the rooted species tree are in newick format and can be viewed using programs such as Dendroscope (http://dendroscope.org/) or FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
OrthoFinder provides a number of options to allow you to incrementally add and remove species.
OrthoFinder allows you to add extra species without re-running the previously computed BLAST searches:
orthofinder -b previous_orthofinder_directory -f new_fasta_directory
This will add each species from the 'new_fasta_directory' to existing set of species, reuse all the previous BLAST results, perform only the new BLAST searches required for the new species and recalculate the orthogroups. The 'previous_orthofinder_directory' is the OrthoFinder 'WorkingDirectory/' containing the file 'SpeciesIDs.txt'.
OrthoFinder allows you to remove species from a previous analysis. In the 'WorkingDirectory/' from a previous analysis there is a file called 'SpeciesIDs.txt'. Comment out any species to be removed from the analysis using a '#' character and then run OrthoFinder using:
orthofinder -b previous_orthofinder_directory
where 'previous_orthofinder_directory' is the OrthoFinder 'WorkingDirectory/' containing the file 'SpeciesIDs.txt'.
The previous two options can be combined, comment out the species to be removed as described above and use the command:
orthofinder -b previous_orthofinder_directory -f new_fasta_directory
This functionality is to be incorporated into the main 'orthofinder' program, replacing the 'trees_from_MSA' utility.
The 'trees_from_MSA' utility will automatically generate multiple sequence alignments and gene trees for each orthogroup generated by OrthoFinder. For example, once OrthoFinder has been run on the example dataset, trees_from_MSA can be run using:
trees_from_MSA orthofinder_results_directory [-t number_of_threads]
This will use MAFFT to generate the multiple sequence alignments and FastTree to generate the gene trees. Both of these programs need to be installed and in the system path.
There are two separate options for controlling the parallelisation of OrthoFinder. The '-t' option should always be used whereas RAM requirements may affect whether you use the '-a' option or not.
-
'-t number_of_threads': This option should always be used. It makes the BLAST searches, the tree inference and gene-tree reconciliation run in parallel. These are all highly-parallelisable and the BLAST searches in particular are by far the most time-consuming task. You should use as many threads as there are cores available.
-
'-a number_of_orthofinder_threads' The remainder of the algorithm, beyond these highly-parallelisable tasks, is relatively fast and efficient and so this option has less overall effect. It is most useful when running OrthoFinder using pre-calculated BLAST results since the time savings will be more noticeable in this case. Using this option will also increase the RAM requirements (see manual for more details).
The '-op' option will prepare the files in the format required by OrthoFinder and print the set of BLAST commands that need to be run.
orthofinder -f fasta_files_directory -op
This is useful if you want to manage the BLAST searches yourself. For example, you may want to distribute them across multiple machines. Once the BLAST searches have been completed the orthogroups can be calculated using the '-b' command as described in Section "Using Pre-Computed BLAST Results".
It is possible to run OrthoFinder with pre-computed BLAST results provided they are in the correct format. They can be prepared in the correct format using the '-op' command and, equally, the files from a previous OrthoFinder run are also in the correct format to rerun using the '-b' option. The command is simply:
orthofinder -b directory_with_processed_fasta_and_blast_results
If you are running the BLAST searches yourself it is strongly recommended that you use the '-op' option to prepare the files first (see Section "Running BLAST Searches Separately"). Should you need to prepare them manually, the required files and their formats are described in the appendix of the PDF Manual (for example, if you already have BLAST search results from another source and it will take too much computing time to redo them).
A set of regression tests are included in the directory 'Tests' available from the github repository. They can be run by calling the script 'test_orthofinder.py'. They currently require version 2.2.28 of NCBI BLAST and the script will exit with an error message if this is not the case.