Philip Turner
Independent Research, December 2023
This literature review was created to investigate computational methods used to study mechanosynthesis. Before conducting it, the author learned that density functional theory (DFT) is the dominant simulation algorithm. The goal is to understand how DFT and SEQM are used in practical mechanosynthesis research. The review should be finished before the author compiles, troubleshoots, or optimizes any quantum mechanics software.
Data:
- Each instance of supercomputer use should be thoroughly documented.
- Estimate quantitative data such as compute power (FLOPS) and compute cost (FLOPs) for each isolated simulation.
- Look for places where lower levels of theory could be used, such as semiempirical quantum mechanics instead of density functional theory.
Insights:
- Typical system sizes used in simulations (number of atoms, number of electrons) and parallelism (number of simulations executing concurrently).
- Inherent lack of parallelism in certain algorithms or at small system sizes (compute power per isolated simulation).
- Rough quantification of energy error for different levels of theory, and what amount of error is tolerable.
The review covers every document published by Robert Freitas, Jr. There are 15 research papers and 17 patent variants. Every research paper will be summarized in depth. Patents will be summarized in final form. An exception is US7687146, summarized from Robert's dedicated web page. Papers are sorted by date of publication; patents are sorted by date of filing.
Papers:
- Theoretical Analysis of a Carbon-Carbon Dimer Placement Tool for Diamond Mechanosynthesis
- A Simple Tool for Positional Diamond Mechanosynthesis, and its Method of Manufacture
- Theoretical Analysis of Diamond Mechanosynthesis. Part I. Stability of C2 Mediated Growth of Nanocrystalline Diamond C(110) Surface
- Theoretical Analysis of Diamond Mechanosynthesis. Part II. C2 Mediated Growth of Diamond C(110) Surface via Si/Ge-Triadamantane Dimer Placement Tools
- Theoretical Analysis of Diamond Mechanosynthesis. Part III. Positional C2 Deposition on Diamond C(110) Surface using Si/Ge/Sn-based Dimer Placement Tools
Patents:
- Atomically-Precise Products and Methods and Systems for Manufacturing the Same
- Systems and Methods for Mechanosynthesis
Robert Freitas first started researching diamond mechanosynthesis in 2003, in collaboration with Ralph Merkle. He published several papers, most in the Journal of Computational and Theoretical Nanoscience. The trail of papers stopped abruptly in 2013. This was the same year that Freitas and Merkle published a precursor to one of the CBN patents. The document, US20130178626, was abandoned after the patent office received no response in 2017.
| Years | Activity |
|---|---|
| 1996–2003 | Nanomedicine, Vol. 1 & 2A |
| 2003–2004 | Mechanosynthesis, Zyvex |
| 2006—2008 | Mechanosynthesis, Nanofactory Collaboration |
| 2010–2012 | Mechanosynthesis, Kazan Federal University |
| 2012–2015 | Nanomedicine, Vol. 2B & 3 (Unpublished) |
| 2016–Present* | Mechanosynthesis, CBN Nano Technologies |
| 2020–2022 | Nanomedicine, Cryostasis Revival |
Rough timeline categorizing Robert Freitas’s activity in published literature. *There is a ~2 year delay between patent filing and granting. Patents created in 2022 will not materialize until roughly 2024.
Literature:
- Ralph C. Merkle, Robert A. Freitas Jr., “Theoretical analysis of a carbon-carbon dimer placement tool for diamond mechanosynthesis,” J. Nanosci. Nanotechnol. 3(August 2003):319-324. PubMed Abstract (HTML) ..... Conference Abstract (HTML) ..... Full Paper (PDF) ..... Full Paper (HTML) ..... Full Paper (PDF)
- Robert A. Freitas Jr., “A Simple Tool for Positional Diamond Mechanosynthesis, and its Method of Manufacture,” U.S. Provisional Patent Application No. 60/543,802, filed 11 February 2004; U.S. Patent Pending, 11 February 2005. Full Paper (HTML, 1.0 MB) ..... See Also (HTML)
- Jingping Peng, Robert A. Freitas Jr., Ralph C. Merkle, “Theoretical Analysis of Diamond Mechanosynthesis. Part I. Stability of C2 Mediated Growth of Nanocrystalline Diamond C(110) Surface,” J. Comput. Theor. Nanosci. 1(March 2004):62-70. Full Text (PDF, 3.1 MB)
- David J. Mann, Jingping Peng, Robert A. Freitas Jr., Ralph C. Merkle, “Theoretical Analysis of Diamond Mechanosynthesis. Part II. C2 Mediated Growth of Diamond C(110) Surface via Si/Ge-Triadamantane Dimer Placement Tools,” J. Comput. Theor. Nanosci. 1(March 2004):71-80. Full Text (PDF, 2.1 MB)
- Jingping Peng, Robert A. Freitas Jr., Ralph C. Merkle, James R. Von Ehr, John N. Randall, George D. Skidmore, “Theoretical Analysis of Diamond Mechanosynthesis. Part III. Positional C2 Deposition on Diamond C(110) Surface using Si/Ge/Sn-based Dimer Placement Tools,” J. Comput. Theor. Nanosci. 3(February 2006):28-41. Full Paper (PDF, 1.0 MB)
- Berhane Temelso, C. David Sherrill, Ralph C. Merkle, Robert A. Freitas Jr., “High-level Ab Initio Studies of Hydrogen Abstraction from Prototype Hydrocarbon Systems,” J. Phys. Chem. A 110 (28 September 2006):11160-11173. ACS Abstract (HTML) ..... PubMed Abstract (HTML) ..... Full Paper (PDF, 0.4 MB)
- Robert A. Freitas Jr., Damian G. Allis, Ralph C. Merkle, “Horizontal Ge-Substituted Polymantane-Based C2 Dimer Placement Tooltip Motifs for Diamond Mechanosynthesis,” J. Comput. Theor. Nanosci. 4(May 2007):433-442. Ingenta Abstract (HTML) ..... Full Paper (PDF, 0.7 MB)
- D.R. Forrest, R.A. Freitas Jr., N. Jacobstein, “Scanning Probe Diamondoid Mechanosynthesis,” IMM White Paper, 1 August 2007. http://www.imm.org/documents/IMM_Roadmap_DMS.pdf
- Berhane Temelso, C. David Sherrill, Ralph C. Merkle, Robert A. Freitas Jr., “Ab Initio Thermochemistry of the Hydrogenation of Hydrocarbon Radicals Using Silicon, Germanium, Tin and Lead Substituted Methane and Isobutane,” J. Phys. Chem. A 111(15 August 2007):8677-8688. ACS Abstract (HTML) ..... PubMed Abstract (HTML) ..... Full Paper (PDF, 0.2 MB)
- Robert A. Freitas Jr., Ralph C. Merkle, “Positional Diamond Mechanosynthesis: Toolset, Reactions, Uses and Products,” U.S. Provisional Patent Application No. 60/970,658, filed 7 September 2007. Abstract (HTML)
- Robert A. Freitas Jr., Ralph C. Merkle, “A Minimal Toolset for Positional Diamond Mechanosynthesis,” J. Comput. Theor. Nanosci. 5(May 2008):760-861. Abstract (HTML) ..... Full Paper (PDF, 6.5 MB)
- Denis Tarasov, Natalia Akberova, Ekaterina Izotova, Diana Alisheva, Maksim Astafiev, Robert A. Freitas Jr., “Optimal Tooltip Trajectories in a Hydrogen Abstraction Tool Recharge Reaction Sequence for Positionally Controlled Diamond Mechanosynthesis,” J. Comput. Theor. Nanosci. 7(February 2010):325-353. Abstract (HTML) ..... Full Paper (PDF, 3.2 MB)
- Robert A. Freitas Jr., “A Simple Tool for Positional Diamond Mechanosynthesis, and its Method of Manufacture,” U.S. Patent No. 7,687,146, issued 30 March 2010. US7687146 (PDF, 1.2 MB, 68 pp) ..... USPTO (HTML) ..... FreePatentsOnline (HTML) ..... FreePatentsOnline (PDF)
- Denis Tarasov, Ekaterina Izotova, Diana Alisheva, Natalia Akberova, Robert A. Freitas Jr., “Structural Stability of Clean, Passivated, and Partially Dehydrogenated Cuboid and Octahedral Nanodiamonds up to 2 Nanometers in Size,” J. Comput. Theor. Nanosci. 8(February 2011):147-167. Abstract ..... Full Paper (PDF, 2.2 MB, uncorrected proof)
- Damian G. Allis, Brian Helfrich, Robert A. Freitas Jr., Ralph C. Merkle, “Analysis of Diamondoid Mechanosynthesis Tooltip Pathologies Generated via a Distributed Computing Approach,” J. Comput. Theor. Nanosci. 8(July 2011):1139-1161. Abstract ..... Full Paper (PDF, 2.1 MB, uncorrected proof) ..... Full Paper (PDF, 1 MB)
- Robert A. Freitas Jr., “Chapter 11. Diamondoid Mechanosynthesis for Tip-Based Nanofabrication,” in Ampere Tseng, ed., Tip-Based Nanofabrication: Fundamentals and Applications, Springer, New York, 2011, pp. 387-400. Purchase Book (Amazon) ..... Full Paper (PDF, 0.4 MB)
- Denis Tarasov, Ekaterina Izotova, Diana Alisheva, Natalia Akberova, Robert A. Freitas Jr., “Structural Stability of Clean and Passivated Nanodiamonds having Ledge, Step, or Corner Features,” J. Comput. Theor. Nanosci. 9(January 2012):144-158. Abstract ..... Full Paper (PDF, 1.9 MB, uncorrected proof)
- Denis Tarasov, Ekaterina Izotova, Diana Alisheva, Natalia Akberova, Robert A. Freitas Jr., “Optimal Approach Trajectories for a Hydrogen Donation Tool in Positionally Controlled Diamond Mechanosynthesis,” J. Comput. Theor. Nanosci. 10(September 2013):1899-1907. Abstract ..... Full Paper (PDF, 1.5 MB, uncorrected proof)
The trail of intellectual property begins with the Nanofactory Corporation, an undocumented entity that sounds similar to the Nanofactory Collaboration (2006). Google Patents reports a transfer of ownership to CBN Nano Technologies circa year 2016. Most of the early patents were filed in 2016, but did not publicly surface until 2018. The following table catalogs each patent in chronological order of submission. Patent revisions and near-duplicates are grouped with the original.
Title |
Date Filed |
ID |
||
Atomically-Precise Products and Methods and Systems for Manufacturing the Same |
Feb 28, 2013 |
US20130178626 |
||
Feb 22, 2016 |
US10308514 |
|||
Jul 7, 2016 |
US10138172 |
|||
May 5, 2017 |
US10197597 |
|||
Nov 7, 2017 |
US10309985 |
|||
Apr 16, 2019 |
US10822230 |
|||
Sep 29, 2020 |
US11155461 |
|||
Systems and Methods for Mechanosynthesis |
May 12, 2016 |
US10072031 |
||
Nov 16, 2016 |
US10067160 |
|||
Nov 13, 2017 |
US10822229 |
|||
Aug 2, 2018 |
US11180514 |
|||
Sep 29, 2020 |
US11148944 |
|||
Sep 14, 2021 |
US11592463 |
|||
Nov 22, 2021 |
US11708384 |
|||
For each entry, the PDF with images is available at: https://patents.google.com/patent/ID
Journal of Nanoscience and Nanotechnology: doi.org/10.1166/jnn.2003.203
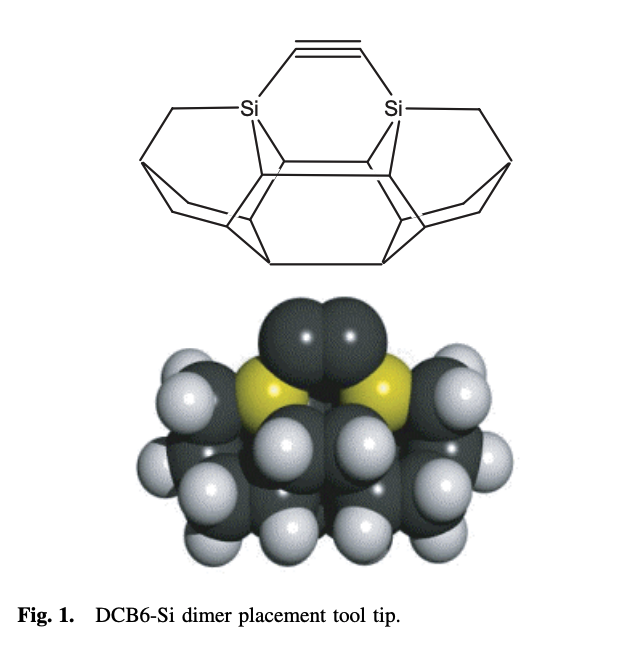
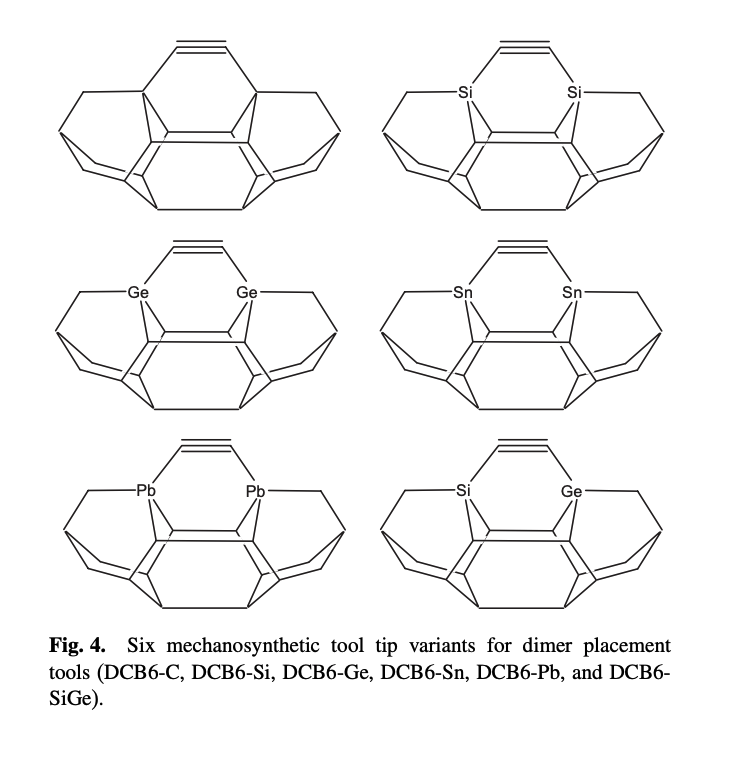
This appears to be a learning experience, where Robert Freitas got acquainted with quantum chemistry techniques. Density functional theory was used to perform energy minimizations on different tooltips. The "DCB", or dicarbon bridge, was established as one of a combinatorial explosion of possible tooltips. It was simple to reason about: three adamantane cages in a rigid diamond lattice. Two carbon atoms were substituted with other group (IV) elements, which form weaker bonds with the feedstock. The germanium variant, "DCB6-Ge", is frequently referenced in later literature.
Tool tip structures examined in the research paper.
| Particle | Quantity | 4-Fold Symmetry |
|---|---|---|
| H | 24 | |
| C | 20 | |
| Si | 2 | |
| Atom | 46 | ~12 |
| Electron | 112 | ~28 |
System: DCB6-Si dimer placement tool tip.
Single-point energy calculations were used to show that undesired bonding arrangements are energetically unfavorable. The tip mostly likely holds the carbon dimer in the horizontal configuration, as designed. An ab initio energy minimization was also performed to test tooltip stability. Single-point energy calculations were used to measure the singlet-triplet energy gap, showing the triplet state is energetically unfavorable. Ab initio molecular dynamics was used to show that the tooltip doesn't dissociate due to thermal motion. The simulation was performed at an extremely high temperature, 1000 K. This is near the thermal decomposition temperature of diamond.
| Algorithm | Software | Usage | Data |
|---|---|---|---|
| Plane-Wave DFT | Gaussian 98 | Single-point energy calculation | 55 energies reported |
| Plane-Wave DFT | Gaussian 98 | Energy minimization | "two minima" |
| MM2 Variant | HyperChem | Energy minimization | "several candidate minima" |
| Plane-Wave DFT | VASP | Molecular dynamics | 5 ps |
| AM1 SEQM | VASP | Molecular dynamics | 200 ps |
Only six months later, Robert would file the first patent on diamond mechanosynthesis.
United States Patent and Trademark Office: US7687146
TODO: Once the entire paper is analyzed, write a summary.
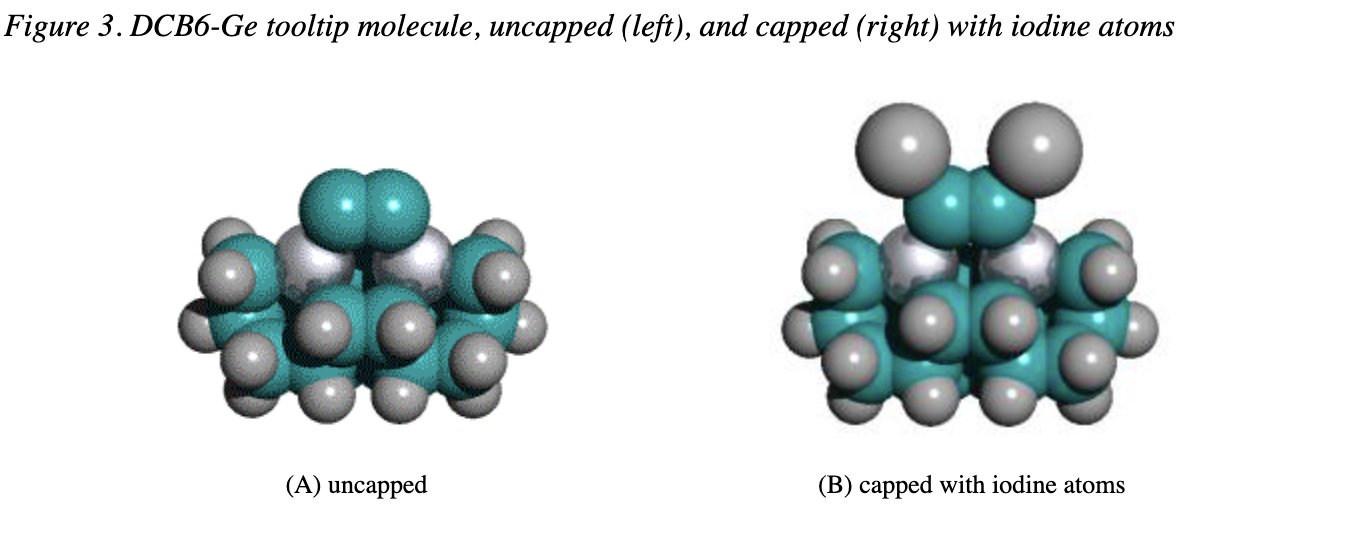
The triadamantane cluster can be synthesized with a myriad of organic chemistry techniques. Variants with silicon and germanium can be created the similarly. While in solution, protective groups must cap the reactive acetylene group. Freitas had to choose between a very large number of possible caps.
Four relevant factors when choosing the capping group:
- Loosely bound, forms the desired geometry, and simple to synthesize.
- Should not spontaneously dimerize in solution phase.
- Should not spontaneously recombine into the adamantane base of other tips.
- Should not spontaneously react with solvent or other molecules involved in synthesis.
Freitas listed each functional group that satisfied each reliability constraint. Iodine, bromine, and a few other groups met all constraints.
Usage of the AM1 semiempirical model:
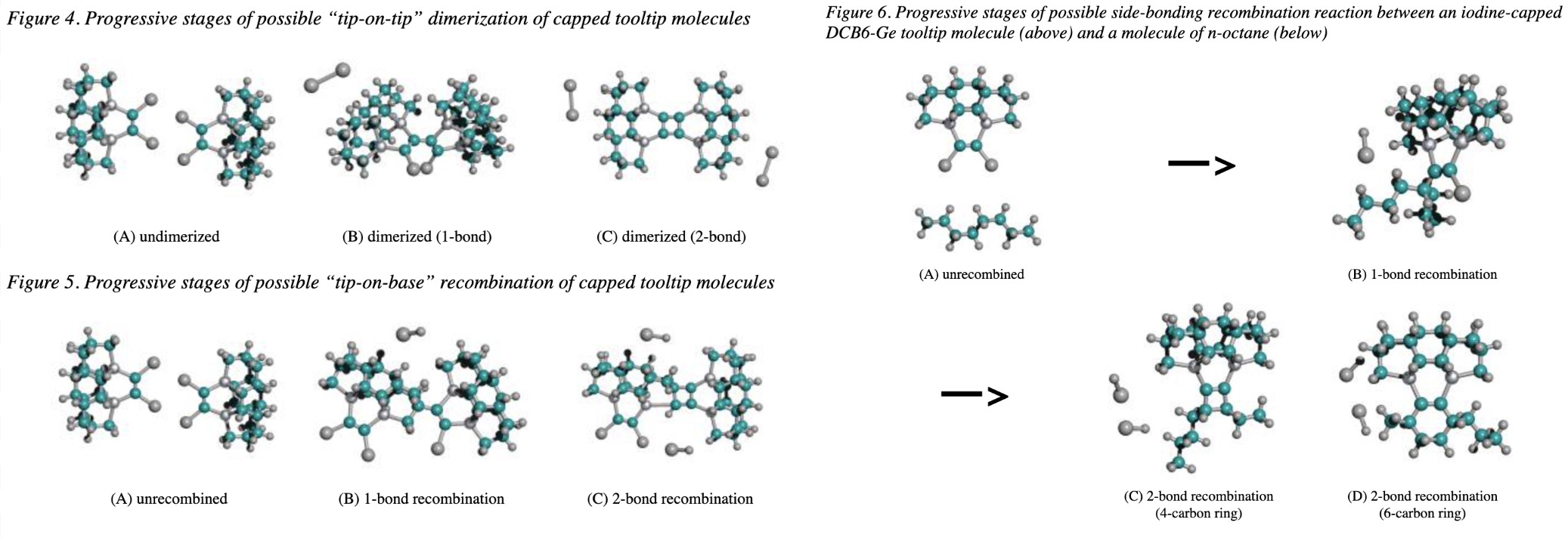
- Energy minimization of different stages of the tip dimerization reaction. PM3 or MNDO semiempirical models were used instead for some cases containing Na, Mg, and Se. All calculations were over the DCB6-Ge variant. ~204 minimized energies were reported in Table 3.
- Another round of analysis was performed for a different dimerization pathway. Here, the dimers don’t attack each other, but some other nearby atoms on the tooltip. The end product is less symmetrical. This reaction was described as “kinetically accessible” due to the size of the simulated energy barrier. ~198 minimized energies were reported in Table 4.
- Studied recombination reactions between DCB6-Ge and octane. ~112 minimized energies were reported in Table 5.
In another Freitas paper, plane-wave DFT was used to study dimerization of uncapped tooltips. Differences in energy between the stages of dimerization were reported. Systems included DCB6-Si and DCB6-Ge. It was not stated whether these were energy minimizations or single-point energy calculations. Electron correlation errors may have created an overly optimistic estimate of resistance to dimerization. Inaccurate correlation energies are an issue with existing DFT simulators, which could be fixed with a more robust exchange-correlation potential (DM21). 6 energy measurements were quoted for symmetric dimerization, and 2 for asymmetric. The actual paper could include many more energies.
| Particle | DCB6-Ge-I2 | Octane |
|---|---|---|
| H | 24 | 18 |
| C | 20 | 8 |
| Ge | 2 | 0 |
| I | 2 | 0 |
| Atom | 48 | 26 |
| Electron | 126 | 50 |
| System | Atoms | Electrons |
|---|---|---|
| DCB6-Si + DCB6-Si | 92 | 224 |
| DCB6-Ge + DCB6-Ge | 92 | 224 |
| DCB6-Ge-I2 + DCB6-Ge-I2 | 96 | 252 |
| DCB6-Ge-I2 + Octane | 74 | 176 |
A suitable surface must be chosen, where the tooltip can be deposited face-down. The deposition density should be ~105 molecules per cm2. This range allows diamond crystals to be grown from the tooltip handles, with enough room to pick them apart with an SPM. The surface should not be capable of growing diamond through CVD. If it were, diamond crystals from the surface would interfere with crystals growing on the tooltips.
AM1 used to measure “energy minima” of 3 different states of the tooltip. Either connected to the surface, or separated from the surface. The latter situation branches into two outcomes: dimer on tooltip (wanted) and dimer on surface (unwanted). All materials showed energetic favorability of not spontaneously dissociating with the surface. Some materials showed a preference to steal the acetylene group from the tooltip. Cu favored this outcome by 18 zJ. However, it was inferred that Ag and Au leave the acetylene on the tooltip. The computed energies were described as “crude estimates”. It is not a “full computational simulation” of the surface interaction.
The tooltip can be attached to the surface in many ways:
- Ionize the tooltip molecule and bombard onto the surface, knocking off caps in the process.
- Attach with physical vapor deposition and remove the caps in a separate step.
- Employ traditional chemical reactions while dissolved in liquid.
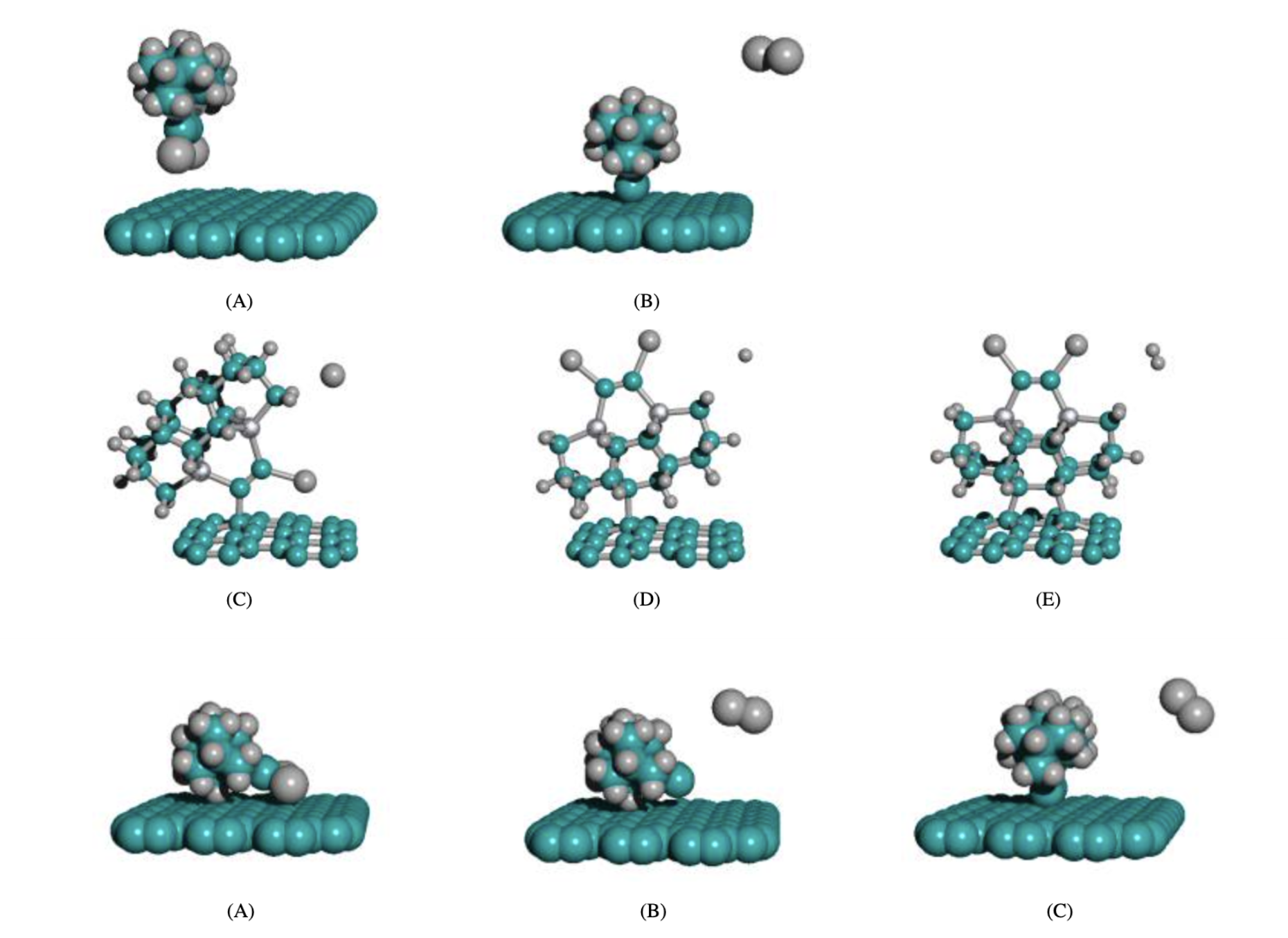
Reactions that may attach the tooltip to the surface. Rendered as attaching to graphene, potentially due to the extreme ease of procedurally generating graphene sheets. Diamond and gold require more complex code to specify in atomic detail.
AM1 was used to energy-minimize the five systems in Figure 7. Absolute energy differences were reported for each system. In addition, the approach angle where the head-on approach would work was estimated. The measure was a range of angles, occupying ~1% of the total solid angle. Geometric hindrance makes the success rate of tooltip collisions only 1%.
AM1 was used to estimate the energy required to remove both caps from the tooltip (PM3 or MNDO for caps with Na, Mg, Se). Energy to remove only one cap is half the energy for both caps. The energies came from a system with only a tooltip, and no surface. 74 single-point energy calculations were reported.
TODO: Add descriptions of sections 2.2.3 and 2.2.4.
Surfaces characterized with quantum mechanics:
- 10-atom cage of group (IV) atoms, not passivated
- 4 copper atoms
- 5 atoms of aluminum oxide
- 3x3 arrangement of graphene unit cells (ambiguous)
| Particle | Adamantane | Copper | Al2O3 | Graphene |
|---|---|---|---|---|
| C | 10 | 0 | 0 | ~14 |
| O | 0 | 0 | 3 | 0 |
| Al | 0 | 0 | 2 | 0 |
| Cu | 0 | 4 | 0 | 0 |
| Atom | 10 | 4 | 5 | ~14 |
| Electron | 40 | ~44 | 36 | ~56 |
| System | Atoms | Electrons |
|---|---|---|
| DCB6-Ge + Adamantane | 56 | 152 |
| DCB6-Ge + Copper | 50 | ~156 |
| DCB6-Ge + Al2O3 | 51 | 148 |
| DCB6-Ge + Graphene | ~60 | ~168 |