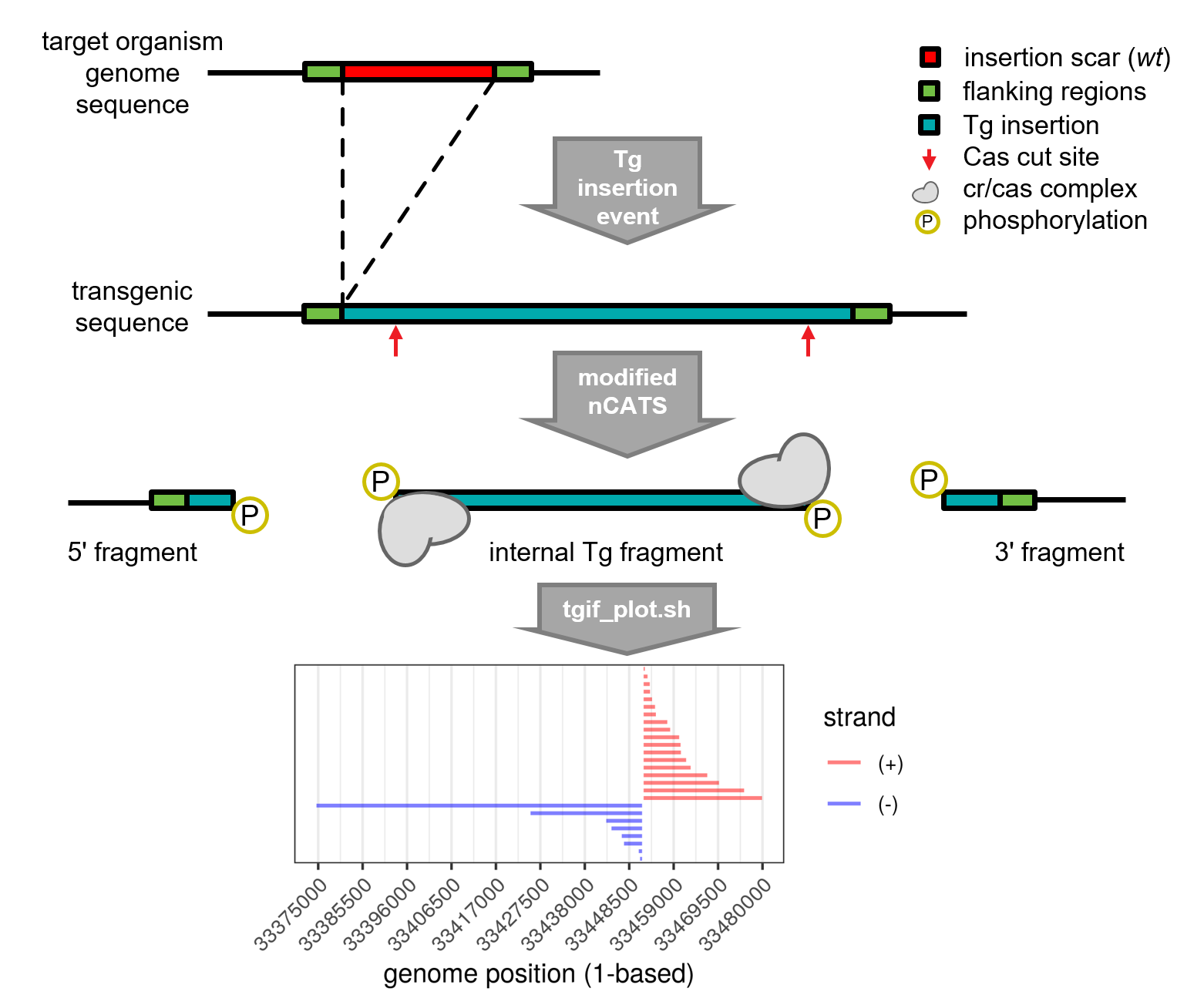
The TgIF algorithm returns a list of probable insertion sites in a target organism. It requires the user to provided a FASTQ file of ONT (Oxford Nanopore Technologies) reads (-f), the reference FASTA of the trans-gene (Tg) vector containing the insertion sequence (-i), and the reference FASTA of the target organism (-r). The algorithm is tailored for ONT reads from a modified nCATS[1] (nanopore Cas9-targeted sequencing) enriched library, however the algorithm will also produce informative results from a FASTQ derived from WGS (shotgun) sequencing libraries. The modified nCATS method is described here, and a brief overview can be found below.
The basic workflow of TgIF is alignment (using minimap2[2]) of reads (-f) to a combined reference of the Tg vector (containing the desired insertion sequence) and target organism (ie. -i and -r are concatenated), and then searching for valleys (or gaps) in the resulting pileup of reads that map to both references at MAPQ>=30. A starting position (ps) of a valley is where the depth (d) at dp=0 and dp-1>0, an ending position (pe) of a valley is where the depth at dp=0 and dp+1>0, and a potential insertion scar is the gap between and including ps and pe.
Pseudocode:
- Due to potential sequence homology between the vector and target genome sequences, reads are aligned to a minimap2 index containing both
- Alignments with MAPQ<30 are discarded, and resulting alignments are then down-selected for the best per read alignment based on highest MAPQ score
- Use changes in read depth as indication of insertion site:
- find gaps in both forward (5' to 3') and reverse (3' to 5') directions
- ensure each forward gap location (+strand) has a match in reverse (-strand)
- find rows where gap length and flanking depths are both >0
- these are the potential insertion sites, likely most will have flanking position depths of 1 and the gap length will be large (>5000 bp)
- Filter and apply confidence estimate to detected sites based on flanking depth and strandedness of aligned reads (see output format col11 for details)
Clone this github repository in a desired local directory and install dependencies available though the apt repo:
git clone https://github.com/jhuapl-bio/TgIF
apt install parallel #required
apt install primer3 #optional
Required dependencies:
Optional dependencies:
- samtools[4]
- R[5] with ggplot2[6] package (if using plotting module)
- Primer3[7] (if using the Primer3 module, symlink the
primer3_corecommand to bin)
After installing the above dependencies symlink executables to tgif's bin directory. An example for minimap2 using which to find the executable path from the globally accessible command, is below:
Symlink (ln -s)example:
mm2path=$(which minimap2)
binpath="/data/apps/tgif/bin/"
ln -s $mm2path $binpath
Help messages:
-h help show help message
-o format show format info for primary tsv output
Required inputs:
-t INT number of threads to GNU parallel over
-f FASTA/Q sequencing reads file (ideally from an ONT nCATS enriched library
-r FASTA fasta reference of target organism (may contain multiple sequences, no linebreaks within each sequence)
Optional inputs:
-i FASTA fasta of plasmid/inserted gene(s), HIGHLY recommended (may only contain a single sequence, no linebreaks within the sequence)
-s y/n output sorted bam of downselected reads for IGV use
-p y/n generate read pileup png with R/ggplot2 per insertion site
f="/data/project/reads.fastq"
r="/data/project/org_reference.fna"
i="/data/project/vector.fasta"
bash tgif_ncats.sh -t 10 -f "$f" -r "$r" -i "$i"
The output from running the command above will be in a directory named for the basename of $f, and in the same parent directory of the input FASTA/Q read file. The output dir would be /data/project/tgif_ncats-reads.fastq/, and the primary output file is insertions_filtered.tgif which is TSV formatted.
All positions are 1-based.
col1 HEADER sequence header from reference [-r] input file where insertion site is predicted
col2 GAP_START position in reference sequence where gap starts (5' end of gap)
col3 PREGAP_DEPTH depth of ("position in col2" - 1)
col4 PREGAP_PSTRANDEDNESS comma separated values: positive strand count, total pregap read count, pos proportion
col5 PREGAP_NSTRANDEDNESS comma separated values: negative strand count, total pregap read count, neg proportion
col6 GAP_END position in reference sequence where gap ends (3' end of gap)
col7 POSTGAP_DEPTH depth of ("position in col6" + 1)
col8 POSTGAP_PSTRANDEDNESS comma separated values: positive strand count, total postgap read count, pos proportion
col9 POSTGAP_NSTRANDEDNESS comma separated values: negative strand count, total postgap read count, neg proportion
col10 GAP_LENGTH length (bp) of deletion (gap, depth zero to zero; col3-col2)
col11 CONFIDENCE [low], [medium], or [high] probability of being an insertion site
low - sites where at least one flanking depth is >1
medium - flanking depths are both >1
high - flanking depths are both >1, and both flanks have 100% opposing strandedness, e.g. if one flank has 100% of reads aligning to the +strand, then 100% of reads from the other align to the -strand (depending on depth, these are almost definitively insertion sites)
*if using shear (WGS) data, 'high' confidence is not as meaningful (and anyways, very unlikely)
Plotting Module
If interested in a visualization of the read pileups at each potential insertion site, use the optional plotting parameter (-p y) in the primary TgIF script (tgif_ncats.sh), or use the separate plotting script (tgif_plot.sh) which takes as input, the path to an output directory of the primary TgIF script (as it requires some intermediate files produced during the TgIF process).
Usage of separate script, using inputs from example above:
bash tgif_plot.sh /data/project/tgif_ncats-reads.fastq/
Primer3 Module
The TgIF Primer3 module (tgif_primer3.sh) is used to design primers for wet lab validation of potential insertion sites identified by the primary TgIF script (tgif_ncats.sh). For each potential insertion site reported in the TSV output file, files named for the reference sequence header and the start and end positions of the gap (col2 and col6) will be output. There will be separate files for the forward and reverse primers (in default Primer3 output format).
These will be designed from a template sequence derived from the target genome reference (-r) 500 bp upstream/downstream of the gap start/end positions, respectively (i.e. col2-500 and col6+500). Since the wild-type sequence will be present in the reference, this part of the template will be masked (ignored) during the design process (i.e. a number of positions equaling the gap length {col10} is masked in the template sequence starting at position 500). It is assumed the user will have a matching reverse or forward primers which are within the insert to match with either the flanking forward and reverse target genome primers output by this module, respectively. These (or any number of other primers output by this module) may be used in separate reactions as validation assays for predicted insertion sites.
Please see Installation section for dependency details.
INPUT ARGUMENTS & SIMPLE EXAMPLE Help messages:
-h help show this message
-f format format of input (insertions_filtered.tgif)
Required inputs:
-t INT number of threads to GNU parallel over
-i TgIF full path to output file of primary tgif algorithm (insertions_filtered.tgif)
-r FASTA fasta reference of target organism used to generate the input file (-i)
Optional inputs:
-g INT if GAP_LENGTH (col10) of insertion site is greater than INT bp (-g), no primers will be generated for the site [default 5000]
Example (following the same primary TgIF script example)
i="/data/project/tgif_ncats-reads.fastq/insertions_filtered.tgif"
r="/data/project/org_reference.fna"
bash tgif_primer3.sh -t 10 -i "$i" -r "$r" -g 2000
The output directory (named primer3_files) will be in the parent directory of the input file (-i), in this case it will be /data/project/tgif_ncats-reads.fastq/primer3_files. Expanding the output directory with tree you can see examples of the naming conventions, and head of the first .for file (containing a list of possible forward primers) gives a flavor of the Primer3 output format (for details, query column headers at http://primer3.org/manual.html):
tree primer3_files/
primer3_files/
├── Chr07-2128004-2128032.for
├── Chr07-2128004-2128032.rev
├── Chr08-33451539-33451905.for
├── Chr08-33451539-33451905.rev
├── log
├── p3record.tgif_aa
├── p3record.tgif_ab
├── parallel.p3
├── tgif
├── tgif_aa
└── tgif_ab
0 directories, 11 files
head primer3_files/Chr07-2128004-2128032.for
ACCEPTABLE LEFT PRIMERS
0-based # self self hair- qual-
# sequence start ln N GC% Tm any_th end_th pin lity
0 AGGAAAGCGAGTCCAAGAGC 185 20 0 55.00 60.037 6.20 0.00 37.65 0.037
1 CCCCGTTTGGATCCTTGGAA 38 20 0 55.00 59.961 14.23 0.00 37.49 0.039
2 CGAGTCCAAGAGCGTGCTAT 192 20 0 55.00 59.899 0.00 0.00 0.00 0.101
3 TTTCCCTTAGGCCCCGTTTG 27 20 0 55.00 60.251 0.00 0.00 0.00 0.251
4 TGGAATGTACCCTGCAGCAA 437 20 0 50.00 59.596 8.66 0.00 0.00 0.404
5 CTTAGGCCCCGTTTGGATCC 32 20 0 60.00 60.466 4.20 4.20 37.49 0.466
6 CCTTAGGCCCCGTTTGGATC 31 20 0 60.00 60.466 0.00 0.00 37.49 0.466
Modified nCATS Method (more details here)
The nCATS method was originally designed for interrogation of a known genomic region. For application to enrichment of reads from an unknown transgene (Tg) insertion site in a target organism, the ends of the Tg are targeted with the guides (CRISPR gRNA), leaving enough of the Tg sequence for specific alignment (gRNA homology indicated by red arrows), and oriented such that after cleavage the cas9 will sit on the internal Tg fragment instead of at each end of the flanking sequences.
These digested fragments will be more amenable for ONT library prep due to the resulting phosphorylation end prep, while library preparation of the internal fragment is hindered by the remaining large cr/cas complex. Additionally, any of these uninformative reads derived from this fragment will be filtered post-alignment since they will only align to the vector sequence. The resulting enrichment of flanking reads helps identify the genomic context of insertion sites via the method described above. Note that (-) strand reads are expected from the 5' fragment and (+) strand reads are expected from the 3' fragment in the example shown in the figure due to the strandedness of the gRNAs.
- please use full paths for file/directory input arguments
- all reference files (-r and -i) should have only 2 lines per sequence (i.e. no newlines [\n or \r] within the sequence)
- there can be multiple sequences in (-r), but there should only be a single sequence in (-i)
- FASTQ input (-f) should be from a modified nCATS enriched library, however may be from a WGS sheared library (however this renders the confidence estimates less useful)
- all 'process comments' output to command line are coming from stderr output (i.e. will not redirect with stdout '>'), they are also printed to a log file
- the insertion sites will be printed to stdout, and are also saved in the output directory in the file
insertions_filtered.tgif - all reported genome positions are 1-based
- output directory will be created where the input read file (-f) exists, named for the basename of the read file (i.e.
tgif_ncats-$(basename $f))
- Gilpatrick, T. et al. Targeted nanopore sequencing with Cas9-guided adapter ligation. Nature Biotechnology 38, 433–438 (2020).
- Li, H. (2018). Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics, 34:3094-3100. doi:10.1093/bioinformatics/bty191
- O. Tange (2011): GNU Parallel - The Command-Line Power Tool, ;login: The USENIX Magazine, February 2011:42-47.
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and 1000 Genome Project Data Processing Subgroup, The Sequence alignment/map (SAM) format and SAMtools, Bioinformatics (2009) 25(16) 2078-9 [19505943]
- R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
- H. Wickham. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016.
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M and Rozen SG. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012 Aug 1;40(15):e115.