SLOctolyzer: Analysis toolkit for automatic segmentation and measurement of retinal vessels on SLO images
Analysis toolkit for automatic segmentation and measurement of retinal vessels for confocal, near infra-red scanning laser ophthalmoscopy (SLO) images.
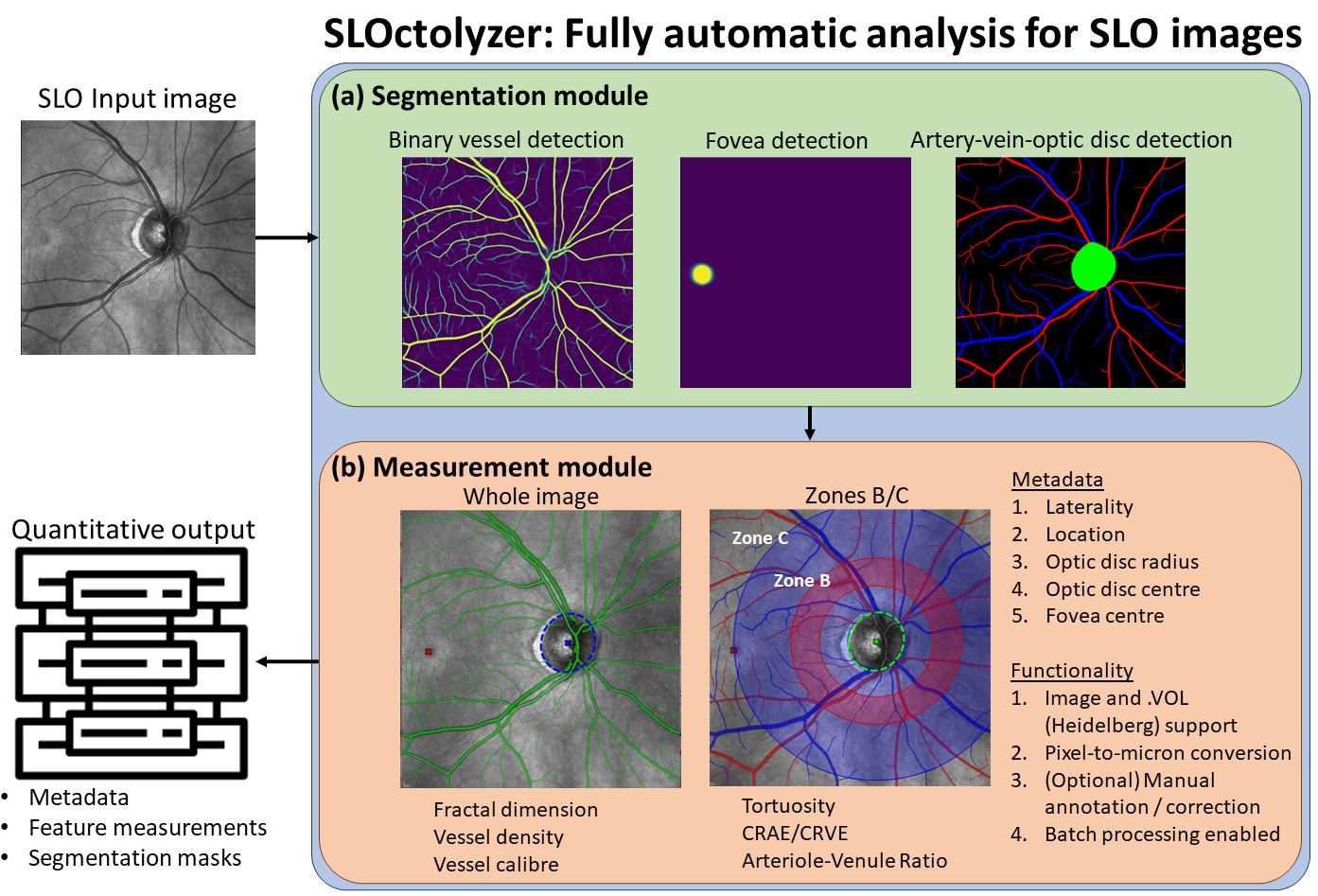
SLOctolyzer is a fully automatic pipeline which is capable of fully characterising the vessels, fovea and optic disc in SLO images. The pipeline utilises fully automatic deep learning methods for segmenting these landmarks, including detection of arteries and veins.
Please find the pre-print describing SLOctolyzer's entire pipeline here, which is currently under review at ARVO's Translational Vision Science and Technology.
SLOctolyzer is also capable of extracting clinically-relevant features of interest of the segmented retinal vessels. The code used to measure retinal vessel features is heavily based on the code produced by Automorph, whose codebase can be found here.
See below for a visual description of SLOctolyzer's analysis pipeline.
.
├── analyze/ # Example data
├── figures/ # Figures for README
├── instructions/ # Instructions, installation, manual annotation help
├── sloctolyzer/ # core module for carrying out segmentation and feature measurement
├── config.txt # Text file to specify where the analysis directory is, and whether to robustly analyse a batch, i.e. ignore unexpected errors and bugs.
├── README.md # This file
└── usage.ipynb # Demonstrative Jupyter notebook to see usage.
- The code found in
sloctolyzer
.
├── sloctolyzer/
├───── measure/ # Feature extraction
├───── segment/ # Segmentation inference modules.
├───── __init__.py
├───── analyse.py # Script to segment and measure a single SLO image and save out results. Can be used interactively via VSCode or Notebooks.
├───── main.py # Wrapper script to run SLOctolyzer from the terminal for batch processing.
└───── utils.py # Utility functions for plotting and processing segmentations.
To get a local copy up follow the steps in instructions/quick_start.txt, or follow the instructions below.
-
Clone the SLOctolyzer repository via
git clone https://github.com/jaburke166/SLOctolyzer.git. -
You will need a local installation of python to run SLOctolyzer. We recommend a lightweight package management system such as Miniconda. Follow the instructions here to download Miniconda for your desired operating system.
-
After downloading, navigate and open the Anaconda Prompt, and individually copy and run each line found in
install.txtto create your own environment in Miniconda and download necessary packages.- Note: if you have a GPU running locally to use SLOctolyzer, line 3 in
instructions/install.txtshould bepip3 install torch torchvision --index-url https://download.pytorch.org/whl/cu121
- Note: if you have a GPU running locally to use SLOctolyzer, line 3 in
Done! You have successfully set up the software to analyse SLO image data!
Now you can:
-
Launch notebooks using
jupyter notebookor jupyter labs usingjupyter laband see the minimal example below so that you can analyse your own SLO data. -
Alternatively, edit the
image_directoryandoutput_directoryoptions inconfig.txtand runpython path\to\SLOctolyzer/sloctolyzer/main.pyto batch process your own dataset.
If you have any problems using this method, please do not hesitate to contact us - see the end of this README for contact details!
# load necessary modules
from sloctolyzer import analyse
from pathlib import Path
# Detect images in analyze/images
paths = []
for type in [".png", ".tif"]:
paths += list(Path("analyze/demo").glob(f"*{type}"))
# Specify path to image, a save_path and scale/location/eye if know. Here, we do not specify
path = paths[0]
save_path = "analyze/output"
scale = None # for (768,768) images this is typically 11.48 for an emmetropic eye
location = None # Can be either "Macula" or "Optic disc" (speech marks inclusive)
eye = None # Can be either "Left" or "Right" (speech marks inclusive)
# Analyse SLO image - saved out in analyze/output/ into a folder whose name is the filename of the image
output = analyse.analyse(path, save_path, scale, location, eye, save_images=1, save_results=1)
# output is a tuple containing metadata as a dataframe, feature measurements as a list of dataframes
# the SLO image, all three segmentation masks and a list of strings for the purposes of logging
Please refer to usage.ipynb for an interactive demonstration of analysing an SLO image. There are some example images used for demonstration in analyze/images.
Below is a short description of the key elements of the analysis pipeline.
At present, SLOctolyzer is vendor neutral and supports most image file formats (with case invariant types tif/tiff/png/jpeg/jpg/bmp). If you are working with imaging devices from Heidelberg Engineering, you may have a RAW export license and thus can extract data with the .vol file format. SLOctolyzer also supports this file format. .vol is helpful for providing essential metadata and image data in one file, permitting measurements to be converted from pixel space to physical space.
See the documents in the instructions folder for details on installing and using SLOctolyzer on your device.
Briefly, SLOctolyzer can be run from the terminal using main.py for analysing batches of images, or using analyse.py individually per image file using your favourite, interactive IDE such as VSCode or Jupyter Notebooks. In the latter, we expect paths (as strings) to images, rather than the pixel arrays as input.
When using the terminal, you can specify certain input parameters using a configuration file, config.txt. Here, you can specify the image_directory and output_directory where your SLO images can be stored and results saved to, respectively.
At present, SLOctolyzer is compatible with Windows and macOS operating systems. Given the compatibility with macOS, it's likely that Linux distributions will also work as expected, although has not been tested explicitly. The installation instructions are also the same across operating systems (once you have installed the relevant Miniconda Python distributions for your own operating system).
Once SLOctolyzer is downloaded/cloned and the conda environment/python packages have been installed using the commands in instructions/install.txt, it is only the file path structures to be aware of when switching between OS, i.e. in the configuration file, config.txt, the image_directory and output_directory should be compatible with your OS.
SLOctolyzer can run reasonably fast using a standard, GPU-less Windows laptop CPU. Even without GPU accelerations, the segmentation inference of all three models only takes around ~11 seconds. Nevertheless, SLOctolyzer is equipped to detect if there is a GPU available (CUDA only, not MPS for macOS currently) to accelerate segmentation inference.
Feature measurement is longer than segmentation inference because:
- Measurements are made for the binary vessel, artery and vein segmentation maps.
- For optic disc-centred SLO images, there are three regions of interest considered making it longer to measure than macula-centred images.
- Image resolution plays a role too, where (768,768) image resolution is quicker than (1536,1536) resolution.
However, based on the example images in analyze/demo, a standard laptop Windows CPU takes
- ~15 seconds for an macula-centred SLO image at (768,768) resolution.
- ~30 seconds for an optic disc-centred SLO image at (768,768) resolution.
- ~100 seconds for an optic disc-centred SLO image at (1536,1536) resolution.
The execution time here may vary dependent on the size of the CPU, and whether GPU acceleration is utilised for segmentation inference, so only act as a rough guide.
At present, the features measured on the SLO image across the whole image are:
- Fractal dimension (dimensionless)
- Vessel perfusion density (dimensionless)
- Global vessel calibre (computed globally in microns or pixels, dependent on whether a conversion factor is specified, see below)
Additionally, for smaller regions of interest, zones B and C, and the whole image, the following measurements will be measured in pixels or microns, dependent on whether the conversion factor is specified (see below):
- Tortuosity density
- Local vessel calibre (similar to average vessel width, but computed and averaged across individual vessel segments)
- CRAE (for detected arteries only) using Knudston's formula
- CRVE (for detected veins only) using Knudston's formula
- arteriolar–venular ratio (AVR), which is CRAE divided by CRVE.
A note on tortuosity: A current limitation of the pipeline surrounds artery and vein map disconnectedness. Artery-vein crossings on the SLO are segmented such that only artery OR vein is classified, not both. This was an oversight at the point of ground truth annotation before model training. Thus, the individual artery/vein maps are disconnected. Unfortunately, this may have an impact on tortuosity density measurements.
At present, macula-centred SLO images have only one region of interest (ROI) which is the whole image.
For optic disc-centred SLO images there are three ROI's used for feature measurement. In the description below, D is the diameter of the optic disc.
- Zone B: This is an annulus ROI centred at the optic disc, which measures between 0.5D and 1D from the disc margin (0.5D - 1D).
- Zone C: This is an annulus ROI centred at the optic disc, which measures from the disc margin to two diameters of the disc away (0 - 2D).
- The whole image.
OCT imaging devices which produce SLO image data provide an exact conversion factor between pixel space and physical space. This can be helpful to convert measurements from pixels into microns (or mm). For Heidelberg Engineering data, each distinct acquisition has their own conversion factor depending on the cllibration of the device during examination (scan focus setting, corneal cuvrature, etc.)
Features such as average_vessel_width, vessel_calibre, CRAE and CRVE are measured in pixels and thus can be converted into microns if the conversion factor is known.
In analyze there is an excel document fname_resolution_location_eye.xlsx where you can store the filenames of the images planned for analysis in the Filename column, and their corresponding conversion factor, or scale, measured in microns per pixel in the Scale columns. You can also include information on the eye type in the Eye column ("Right" or "Left"), and the location of the scan ('Macula'- or 'Optic disc'-centred) using the Location column.
If you do not care or have access to the conversion factor for any image, you can leave the Scale cell blank. Similarly for cells in the Location and Eye columns if this information is not known.
In fact, you can ignore the document entirely and SLOctolyzer will still run, and will by default only output pixel-unit measurements, and try infer the eye type and location based on the detected locations of the optic disc centre and fovea. Sanity checking the eye and location classification is recommended by inspecting the "segmentations" directory in the output folder after running the software.
-SLO images are typically either centred at the fovea or centred at the optic disc. There are standard regions of interest defined to analyse these different types of scans (see above). You can use the excel document fname_resolution_location_eye.xlsx found in analyze to specify the location of each image (in the Location columns). This is not compulsory, as the pipeline does support automatic detection of the location of the SLO scan if the location is not provided by the user. However, it remains to be seen how robust this detection is, users be aware! This goes the same for detecting the eye type (Right or Left).
We do not have any automatic functionality within SLOctolyzer to correct any vessel segmentation errors. Thus, we rely on the user to identify any visible problems with vessel classification.
However, we do provide functionality to correct retinal vessel and optic disc segmentation via ITK-Snap. There are instructions on using ITK-Snap for manual annotations in instructions/manual_annotations which describe how to setup ITK-Snap and use it to correct the binary vessel mask, and also the artery-vein-optic disc segmentation masks.
Once the corrected segmentations are saved out as .nii.gz files in the same folder with the original .png segmentation mask(s), the pipeline can be run again and SLOctolyzer should automatically identify these additional manual annotations and re-compute the features!
If you have any issues with running this toolkit on your own device, please contact us (see end of README for contact email).
This project and software package is an evolving toolkit, so we are expecting unexpected errors to crop up on use-cases which the developers have not foreseen. We hope this pipeline will continue to be adapted to the needs of the end-user, so we welcome any and all feedback!
At the moment, setting robust_run to 1 will ensure that batch-processing does not fail if an unexpected error is caught. In fact, if an exception is found, details on the error in terms of it's type and full traceback are saved out in the process log (as well as printed out on the terminal/IDE) so the end-user may interrogate the codebase further and understand the source of the error.
If you are interested in OCT (and SLO) image analysis, check this repoistory out:
- OCTolyzer: A fully automatic analysis toolkit for segmentation and feature extracting in OCT and SLO data.
If you are interested in automatic choroid analysis in OCT B-scans specifically, check these repositories out:
- Choroidalyzer: A fully automatic, deep learning-based toolkit for choroid region and vessel segmentation, and fovea detection in OCT B-scans.
- DeepGPET: fully automated choroid region segmentation in OCT B-scans.
- MMCQ: A semi-automatic algorithm for choroid vessel segmentation in OCT B-scans based on multi-scale quantisation, histogram equalisation and pixel clustering.
If you are interested in colour fundus photography (CFP) image analysis, check this repository out:
- Automorph: Automated retinal vascular morphology quantification via a deep learning pipeline.
The contributors to this method and codebase are:
- Jamie Burke (Jamie.Burke@ed.ac.uk)
If you wish to use this toolkit please consider citing our work using the following BibText
@article{burke2024sloctolyzer,
title={SLOctolyzer: Fully automatic analysis toolkit for segmentation and feature extracting in scanning laser ophthalmoscopy images},
author={Burke, Jamie and Gibbon, Samuel and Engelmann, Justin and Threlfall, Adam and Giarratano, Ylenia and Hamid, Charlene and King, Stuart and MacCormick, Ian JC and MacGillivray, Tom},
journal={arXiv preprint arXiv:2406.16466},
year={2024}
}