Automatically generate products from reactant SMILES.
$ python3 generate_products.py [file.csv]
$ python3 generate_images.py [file_out.csv]
$ python3 fix_errors.py [file_out.csv]
This generates a separate file with the suffix [file]_out.csv (any
sanitization errors encountered will be written to [file]_err.csv). To
generate images, run generate_images.py on this output file.
- Python 3.9.4
- RDKit 2021.03.1b1
transforms.py consists primarily of two dictionaries: one for nucleophiles
and one for electrophiles. Each nucleophile/electrophile is represented as a
SMARTS string, for both reactant and product:
nucleophiles = {
"alkene": ["[C:1]=[CX3:2]", "[C+:1][CX3:2]"],
...
}
electrophiles = {
"C+": ["[CX3+:1]", "[C+0:1]"],
...
}
Since all transformations involve only one nucleophile and electrophile, a comprehensive set of all possible reaction SMARTS (limited to simple SN2-type single bond formations) can be generated by iterating through both dictionaries and performing simple string concatenation:
# electrophile atom indices are modified since
# no two atoms can share the same index
transformations = {
"[C:1]=[CX3:2].[CX3+:11]>>[C+:1][CX3:2][C+0:11]",
["alkene", "C+"],
...
}
This allows a large collection of reaction SMARTS to be built up with little manual work.
smartjoin.py accepts two reactants (represented as SMILES) and determines the
most likely product by:
- iterating through the entire set of transformations,
- attempting to find each substructure match,
- applying the corresponding transformation, and
- attempting to sanitize all possible products.
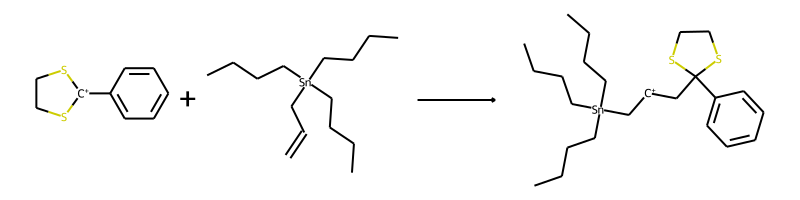
$ python3 smartjoin.py 'C1CS[C+](S1)C1=CC=CC=C1.CCCC[Sn](CCCC)(CCCC)CC=C
1 matching transformations found
1: ['alkene', 'C+'], 1 products
Up to 2 good products found
C1CS[C+](S1)C1=CC=CC=C1.CCCC[Sn](CCCC)(CCCC)CC=C>>CCCC[Sn](C[CH+]CC1(C2=CC=CC=C2)SCCS1)(CCCC)CCCC
['alkene', 'C+']
Sanitized products obtained from complex transformations typically take precedence over those obtained from less complex ones, and the first product obtained is assumed to be the correct one. Unsanitized products are usually a sign of an incorrectly devised SMARTS string, which should be fixed.
This iteration process can be fairly CPU-intensive and is far from optimised, but it seems to work well enough for a relatively small number of nucleophiles/electrophiles (currently less than 30 each).
generate_products.py simply parses a csv file and passes every reactant
SMILES string that it finds to smartjoin.py. The results are written to a new
csv file, which can then be passed to generate_images.py to generate images
for all reactions.
As an example, the resulting image of the above reaction should look like this:
Since it is difficult to guarantee that the correct transformation is applied,
false substructure matches will typically be obtained. The generated images
should then be inspected for errors, and any incorrectly generated reaction
SMILES can be fixed manually with fix_errors.py. This replaces the SMILES
string in the output file, as well as the corresponding image.
- Any transformations more complex than a simple single bond formation
- Automatic creation of new SMARTS (nucleophiles and electrophiles have to be manually added)
- Reactions with more than 2 reactants
- Concatenate output reaction SMILES to a copy of the input file
- This can be achieved with
paste -d, IN.csv <(< OUT.csv awk -F, '{print $NF}') > IN_COPY.csv
- This can be achieved with
- Make SMARTS stricter to avoid false positive matches
- Programmatically detect false positive matches, and give some kind of warning
generate_images.py: accept reactant SMILES as inputgenerate_products: allow user to choose transformation (and show image afterwards)- Create a comprehensive test suite (current test is based on an older approach)