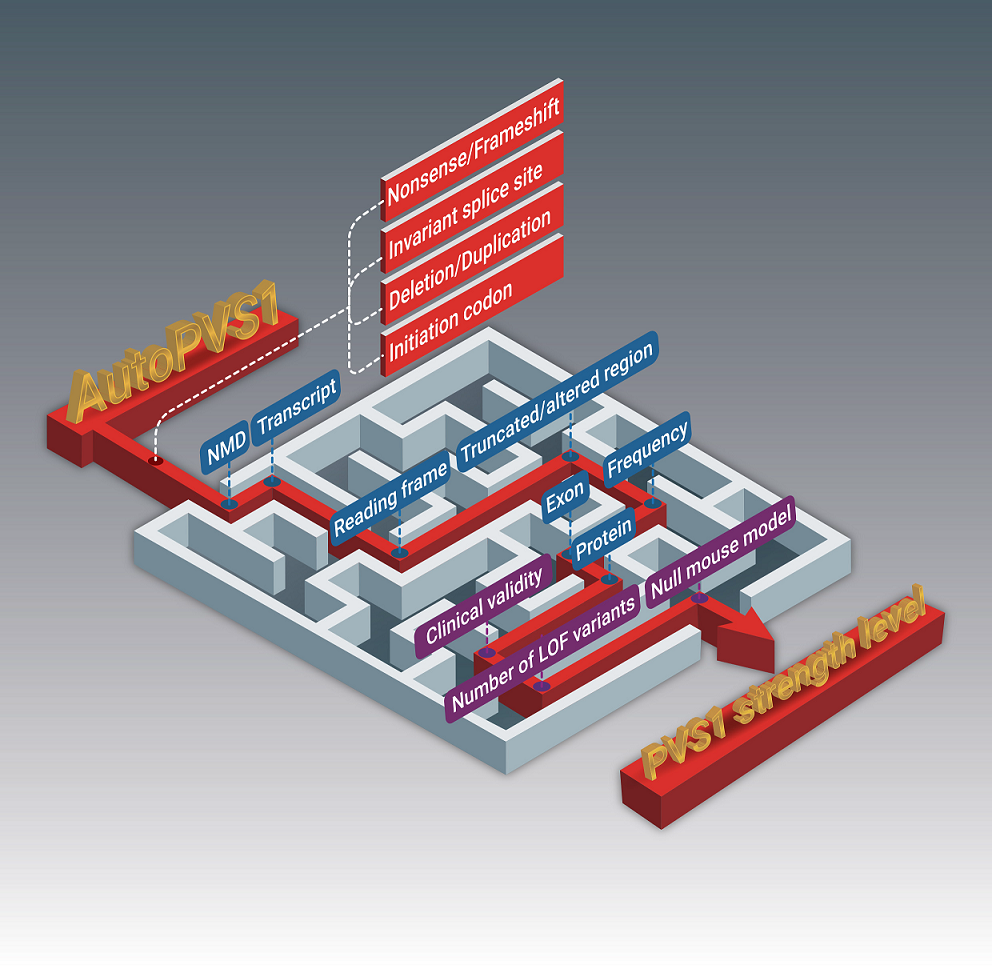
An automatic classification tool for PVS1 interpretation of null variants.
A web version for AutoPVS1 is also provided: https://autopvs1.genetics.bgi.com
🎨 AutoPVS1 is now compatible with hg19/GRCh37 and hg38/GRCh38.
AutoPVS1 use VEP to determine the effect of variants (SNVs, insertions, deletions, CNVs) on genes, transcripts, and protein sequence. To get HGVS name for the variant, indexed_vep_cache (homo_sapiens_refseq 104_GRCh37 and 104_GRCh38) and fasta files are required.
git clone https://github.com/Ensembl/ensembl-vep.git
cd ensembl-vep
git pull
git checkout release/104
perl INSTALL.plVEP cache and faste files can be automatically downloaded and configured using INSTALL.pl. You can also download and set up them manually:
r=104
FTP='ftp://ftp.ensembl.org/pub/'
# indexed vep cache
cd $HOME/.vep
wget $FTP/release-${r}/variation/indexed_vep_cache/homo_sapiens_refseq_vep_${r}_GRCh38.tar.gz
wget $FTP/release-${r}/variation/indexed_vep_cache/homo_sapiens_refseq_vep_${r}_GRCh37.tar.gz
tar xzf homo_sapiens_vep_${r}_GRCh37.tar.gz
tar xzf homo_sapiens_vep_${r}_GRCh38.tar.gz
# fasta
cd $HOME/.vep/homo_sapiens_refseq/${r}_GRCh37/
wget $FTP/grch37/current/fasta/homo_sapiens/dna/Homo_sapiens.GRCh37.dna.primary_assembly.fa.gz
tar xzf Homo_sapiens.GRCh37.dna.primary_assembly.fa.gz
cd $HOME/.vep/homo_sapiens_refseq/${r}_GRCh38/
wget $FTP/current_fasta/homo_sapiens/dna/Homo_sapiens.GRCh38.dna.primary_assembly.fa.gz
tar xzf Homo_sapiens.GRCh38.dna.primary_assembly.fa.gzSamtools provides a function “faidx” (FAsta InDeX), which creates a small flat index file “.fai” allowing for fast random access to any subsequence in the indexed FASTA file, while loading a minimal amount of the file in to memory.
pyfaidx module implements pure Python classes for indexing, retrieval, and in-place modification of FASTA files using a samtools compatible index.
maxentpy is a python wrapper for MaxEntScan to calculate splice site strength. It contains two functions. score5 is adapt from MaxEntScan::score5ss to score 5' splice sites. score3 is adapt from MaxEntScan::score3ss to score 3' splice sites.
maxentpy is already included in the autopvs1.
pyhgvs provides a simple Python API for parsing, formatting, and normalizing HGVS names. But it only supports python2, I modified it to support python3 and added some other features. It is also included in the autopvs1.
autopvs1/config.ini
[DEFAULT]
vep_cache = $HOME/.vep
pvs1levels = data/PVS1.level
gene_alias = data/hgnc.symbol.previous.tsv
gene_trans = data/clinvar_trans_stats.tsv
[HG19]
genome = data/hg19.fa
transcript = data/ncbiRefSeq_hg19.gpe
domain = data/functional_domains_hg19.bed
hotspot = data/mutational_hotspots_hg19.bed
curated_region = data/expert_curated_domains_hg19.bed
exon_lof_popmax = data/exon_lof_popmax_hg19.bed
pathogenic_site = data/clinvar_pathogenic_GRCh37.vcf
[HG38]
genome = data/hg38.fa
transcript = data/ncbiRefSeq_hg38.gpe
domain = data/functional_domains_hg38.bed
hotspot = data/mutational_hotspots_hg38.bed
curated_region = data/expert_curated_domains_hg38.bed
exon_lof_popmax = data/exon_lof_popmax_hg38.bed
pathogenic_site = data/clinvar_pathogenic_GRCh38.vcfYou can specify the vep cache directory to use, default is $HOME/.vep/
hg19.fa is downloaded from UCSC hg19.fa.gz and indexed with samtools faidx
hg38.fa is downloaded from NCBI GRCh38_no_alt_analysis_set.fna.gz and indexed with samtools faidx
Note: the chromesome name in fasta files should have chr prefix
from autopvs1 import AutoPVS1
demo = AutoPVS1('13-113803407-G-A', 'hg19')
demo2 = AutoPVS1('13-113149093-G-A', 'hg38')
if demo.islof:
print(demo.hgvs_c, demo.hgvs_p, demo.consequence, demo.pvs1.criterion,
demo.pvs1.strength_raw, demo.pvs1.strength)
# GRCh37 and GRCh38 is also supported
demo = AutoPVS1('13-113803407-G-A', 'GRCh37')
demo2 = AutoPVS1('13-113149093-G-A', 'GRCh38')Please see https://autopvs1.genetics.bgi.com/faq/
Users may freely use the AutoPVS1 for non-commercial purposes as long as they properly cite it.
This resource is intended for research purposes only. For clinical or medical use, please consult professionals.
📝citation: Jiale Xiang, Jiguang Peng, Samantha Baxter, Zhiyu Peng. (2020). AutoPVS1: An automatic classification tool for PVS1 interpretation of null variants. Hum Mutat 41, 1488-1498. (Editor's choice and cover article)