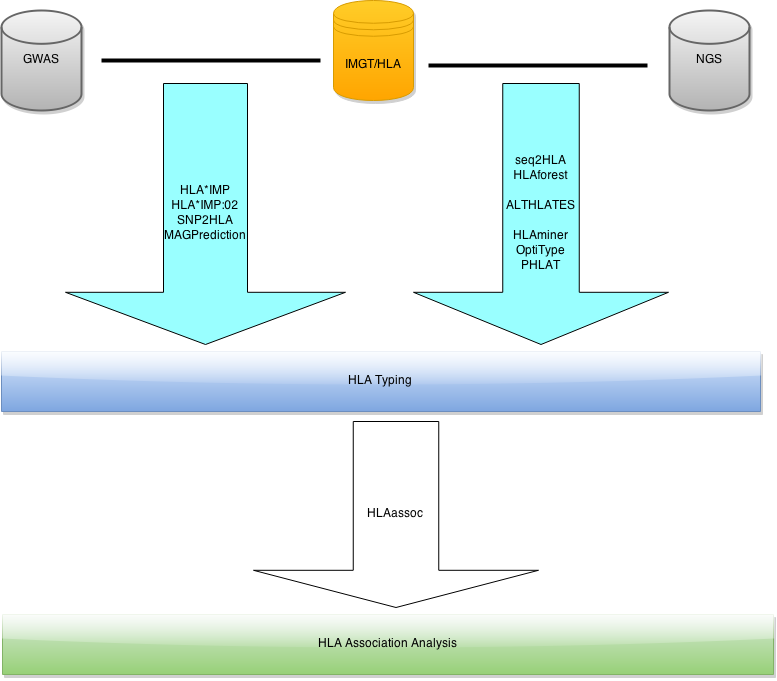
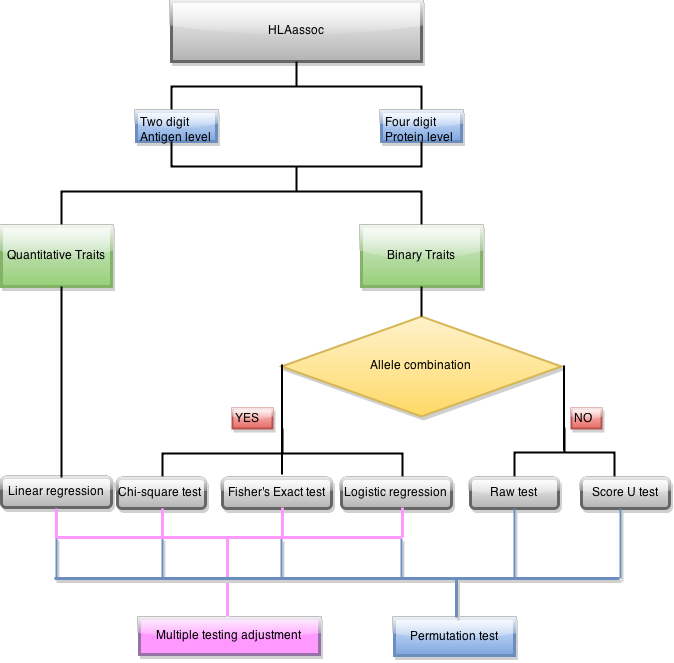
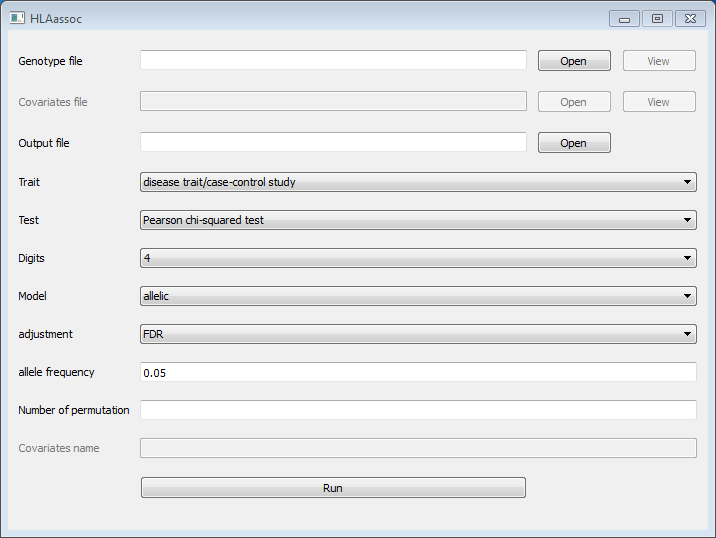
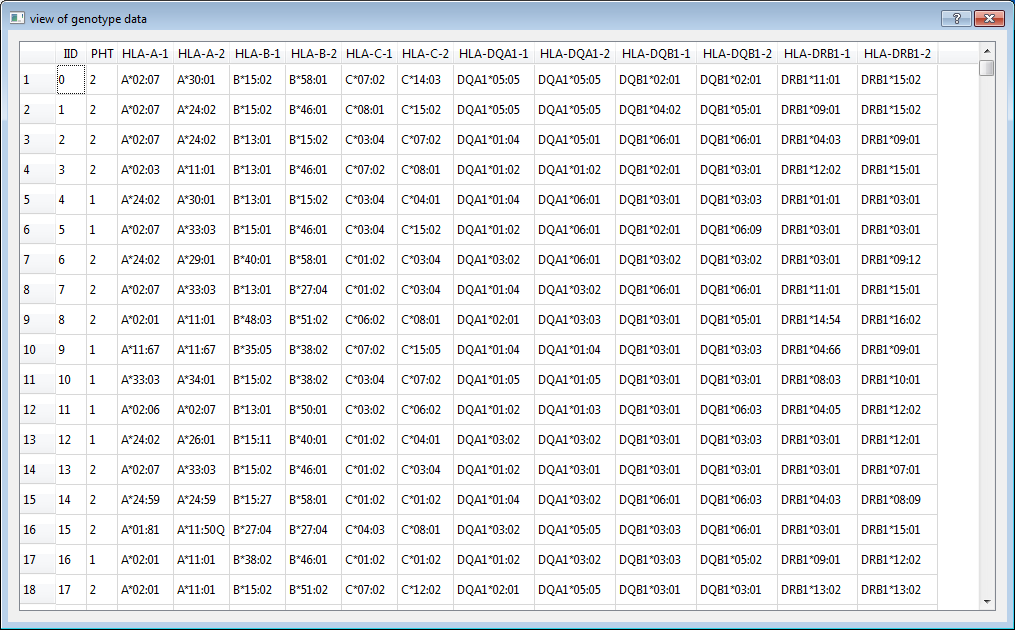
This project has been deprecated, please use PyHLA instead!
HLAassoc: Tests for association between disease and HLA alleles.
0. News
- V1.7 (15 Apr 2015): optimize code and add FDR_BY
- V1.6 (25 Mar 2015): add score test
- V1.5 (19 Mar 2015): add GUI
- v1.4 (12 Mar 2015): add raw test
- v1.3 (10 Mar 2015): add allele frequency to output
- v1.2 (9 Mar 2015): permutation test was added
- v1.1 (16 Feb 2015): linear and logistic regression were added
- v1.0 (23 Jan 2015): initial release.
1. Introduction
HLAassoc provides several methods for association analysis between HLA alleles and diseases.
2. Requirement
- Python 2.7
- pandas
- SciPy
- StatsModels
- PyQt4 (If you want to use the GUI)
3. Installation
Note: There are several free scientific python distributions such as Anaconda and Enthought Canopy which are already integrated the core scientific analytic and scientific Python packages such as SciPy, pandas, StatsModels and PyQt4. To make your life easier, I suggest you install Anaconda or Enthought Canopy and then you do not need to install other modules manually. If you have installed Anaconda or Enthought Canopy, please skip section 3.1 and 3.2.
- 3.1 Install Python 2.7
If you don't already have Python installed. You can follow this guild to install it.
- 3.2 Install Python modules
Please follow this guild to install PyQt4, if you want to use the GUI.
sudo pip install pandas
sudo pip install git+http://github.com/scipy/scipy/
sudo pip install statsmodels
- 3.3 Download HLAassoc
The latest HLAassoc is available here.
4. Options
python HLAassoc.py -h
usage: HLAassoc.py [-h] [-v] -i FILE [-d {2,4,6}] [-m {allelic,dom,rec}]
[-t {chisq,fisher,logistic,linear,raw,score}] [-c COVAR]
[-n COVARNAME] [-f FREQ] [-a {FDR,FDR_BY,Bonferroni,Holm}]
[-o OUT] [-V {False,True}] [-p PERM] [-s SEED]
HLA Association Analysis
optional arguments:
-h, --help show this help message and exit
-v, --version show program's version number and exit
-i FILE, --file FILE input file
-d {2,4,6}, --digits {2,4,6}
digits to test, default 4
-m {allelic,dom,rec}, --model {allelic,dom,rec}
genetic model, default allelic
-t {chisq,fisher,logistic,linear,raw,score}, --test {chisq,fisher,logistic,linear,raw,score}
statistical test method, default chisq
-c COVAR, --covar COVAR
covariants file
-n COVARNAME, --covarname COVARNAME
select a particular subset of covariates
-f FREQ, --freq FREQ minimal frequency, default 0.05
-a {FDR,FDR_BY,Bonferroni,Holm}, --adjust {FDR,FDR_BY,Bonferroni,Holm}
p value correction, default FDR
-o OUT, --out OUT output file
-V {False,True}, --print {False,True}
print output to screen
-p PERM, --perm PERM number of permutation
-s SEED, --seed SEED random seed
4.1) HLA types file (-i or --file)
The input file is a white-space (space or tab) delimited file. The first two columns are mandatory: Individual ID and Phenotype. The Individual IDs are alphanumeric and should uniquely identify a person. The second column is phenotype which can be either a quantitative trait or an affection status. Affection status should be coded as 1 and 2 for unaffected and affected, respectively.
HLA types (column 3 onwards) should also be white-space delimited. Every gene must have two alleles specified. All alleles (see Nomenclature of HLA Alleles) do not need to have the same digits. However, if you want to test association at 4 digits, all alleles should have at least 4 digits resolution. Missing genotype is denoted as NA.
No header row should be given. For example, here are two individuals typed for 6 genes (one row = one person):
0001 2 A*02:07:01 A*11:01:01 B*51:01:01 B*51:01:01 C*14:02:01 C*14:02:01 DQA1*01:04:01 DQA1*01:04:01 DQB1*03:03:02 DQB1*05:02:01 DRB1*07:01:01 DRB1*14:54:01
0002 1 A*24:02:01 A*33:03:01 B*15:25:01 B*58:01:01 C*03:02:02 C*04:03 NA NA DQB1*03:01:01 DQB1*03:01:01 DRB1*03:01:01 DRB1*12:02:01
Note: There are one case and one control. The six genes are: HLA-A, HLA-B, HLA-C, HLA-DQA1, HLA-DQB1 and HLA-DRB1. Each gene has two columns.
Note: Individual 0002 does not have HLA types for HLA-DQA1 (two NA). All alleles have six digits resolution except that one allele of HLA-C of individual 0002 only has four digits resolution. It is fine if we only want to test association at two or four digits resolution.
Note: The allele name contains the HLA prefix, but the allele names in the above example do not have the HLA prefix. Allele names have the HLA prefix can also be used as input. e.g. A*02:07:01 A*11:01:01 is the same as HLA-A*02:07:01 HLA-A*11:01:01.
4.2) Digits resolution (-d or --digits)
Test of association using two digits, four digits or six digits. When two was used, alleles such as A*02:01 and A*02:06 will be combined as A*02. Default value is 4.
4.3) Genetic model to test (-m or --model)
When Pearson chi-squared test or Fisher's exact test was used, three genetic models can be specified.
allelic compares one allele against the others group together
dom compares individuals carry one allele against individuals do not carry it
rec compares individuals carry homozygous of one allele against other individuals
Default value is allelic.
Note: --model only effect when --test chisq or --test fisher is specified.
4.4) Methods for association test (-t or --test)
chisq Pearson chi-squared test (For disease traits, 2 x 2 coningency table)
fisher Fisher's exact test (For disease traits, 2 x 2 coningency table)
logistic logistic regression (For disease traits)
linear linear regression (For quantitative traits)
raw Pearson chi-squared test (For disease traits, 2 x m coningency table)
score Score test proposed by Galta (2005) et al.
When linear or logistic regression was used, assume A*01:01 is the test allele, then A*01:01 A*01:01 is code as 2, A*01:01 A*01:02 is code as 1, and A*01:02 A*01:03 is code as 0.
Default value is chisq.
4.5) Covariates file (-c or --covar)
One or more covariates can be included in linear and logistic regression.
The covariates file is a white-space (space or tab) delimited file. The first row is header. Row 2 onwards contain the individual ID (IID) and measures of several traits. Each row for one individual. The first column is IID and column 2 onwards contain measures of several traits. Each column for one trait.
For example, here are two individuals with three traits:
IID age sex bmi
0001 28 1 20.70
0002 23 0 16.29
Note: Name of trait should not include any white-space.
Note: --covar only effect when --test linear or --test logistic is specified.
Note: The order of individuals in covariates file does not have to be the same as the genotype input file. The number of individuals in covariates file also does not have to be the same as the genotype input file. Only the common individuals of both files were included in the analysis.
4.6) Covariates name (-n or --covarname)
To select a particular subset of covariates, use --covarname covarnames command.
covarnames is a string of trait names (in the header row of covariates file) concatenate with comma(,).
For example,
--covar cov.txt # use all covariates in cov.txt
--covar cov.txt --covarname bmi # only use `bmi`
--covar cov.txt --covarname age,bmi # use both `age` and `bmi`
--covar cov.txt --covarname age,sex,bmi # use all three covariates
Note: if --covarname covarnames command is not specified, all covariates in cov.txt will be used.
4.7) Minimal allele/allele group frequency (-f or --freq)
A value between 0 and 1. Only alleles/allele groups have frequency higher than this threshold will be included in association analysis. Default value is 0.05. For quantitative traits, allele frequency in the whole dataset is used to compare with the threshold. For disease traits, one allele will be included if its frequency in case or control is higher than the threshold.
4.8) Adjustment for multiple testing (-a or --adjust)
Bonferroni Bonferroni single-step adjusted p-values
Holm Holm (1979) step-down adjusted p-values
FDR Benjamini & Hochberg (1995) step-up FDR control
FDR_BY Benjamini & Yekutieli (2001) step-up FDR control
4.9) Permutation (-p or --perm)
Number of permutation will be performed.
For each permutation run, a simulated dataset is constructed from the original dataset by randomizing the assignment of phenotype status among individuals. The same individuals are used, maintaining the same LD structure and the original case/control ratio.
4.10) Output file name (-o or --out)
Default value is hlaassoc.txt.
4.11) Print output to screen (-V or --print)
Default value is False.
4.12) Random seed (-s or --seed)
A number used to initialize the basic random number generator. By default, the current system time is used.
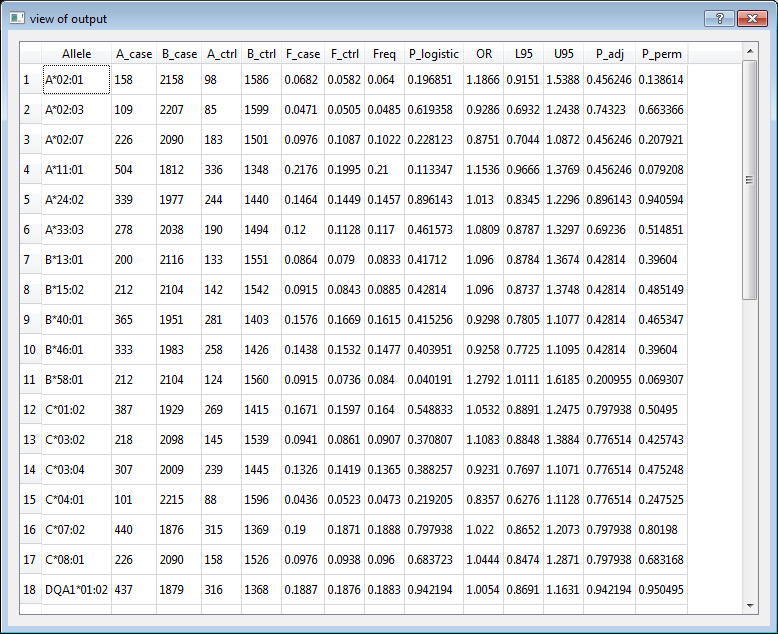
5. Output
Output contains several fields depend on which commands were used.
Allele Allele name
Gene Gene name
A_case Count of this allele in cases
B_case Count of other alleles in cases
A_ctrl Count of this allele in controls
B_ctrl Count of other allele in controls
F_case Frequency of this allele in cases
F_ctrl Frequency of this allele in controls
Freq Frequency of this allele in cases and controls
Chisq Chi-square
DF Degree of freedom
P_chisq P-value for Pearson's chi-squared test
P_Fisher P-value for Fisher's exact test
P_logistic P-value for logistic regression
P_linear P-value for linear regression
U Score test U
OR Odds ratio
beta Regression coefficient
L95 Lower bound of 95% confidence interval for odds ratio or regression coefficient
U95 Upper bound of 95% confidence interval for odds ratio or regression coefficient
P_adj Multiple testing adjusted p value
P_perm P-value for permutation test
5.1 --test chisq or --test fisher
For each allele, a 2 X 2 coningency table contains the count of this allele and the count of the other alleles in the same gene in cases and controls was created. The total number of test is the number of alleles have frequency in cases or controls higher the the threshold specified by option --freq or -f.
The output includes: Allele, A_case, B_case, A_ctrl, B_ctrl, F_case, F_ctrl, Freq, OR, L95, U95, P_adj. The output of Pearson's chi-squared test also includes: Chisq, DF, P_chisq. The output of Fisher's exact test also includes: P_Fisher. When --perm is used, another column P_perm is added to the output.
5.2 --test logistic or --test linear
For each allele, one individual will be coded as 2, 1, 0, if the individual has two copies, one copy, and zero copy of this allele, respectively. The total number of test is the number of alleles have frequency in cases or controls higher the the threshold specified by option --freq or -f.
The output includes: Allele, A_case, B_case, A_ctrl, B_ctrl, F_case, F_ctrl, Freq, L95, U95, P_adj. The output of logistic regression also includes: OR, and P_logistic. The output of linear regression also includes: beta and P_linear. When --perm is used, another column P_perm is added to the output.
5.3 --test raw
Raw test performs a Pearson's chi-squared test on the 2 x m contingency tables for each gene. m is the number of alleles have frequency in cases or controls higher the the threshold specified by option --freq or -f.
The output includes: Gene, Chisq, DF, P_chisq. When --perm is used, another column P_perm is added to the output.
5.3 --test score
Score test calculated the score test U using the formula proposed by Galta et al. (2005). The output includes: Gene, and U. When --perm is used, another column P_perm is added to the output.
6. Usage
python HLAassoc.py -h
python HLAassoc.py --help
python HLAassocGUI.py (start the GUI)
6.1) Disease trait (Case/Control Study)
- Pearson's chi-squared test
The following three commands are equivalent. They perform the Pearson's chi-squared test for each allele that has frequency higher than 0.05 in cases or controls at 4 digits resolution. FDR was used to adjust the p value for multiple testing. Do not write the output to the screen. Save the output to hlaassoc.txt.
python HLAassoc.py --file input0.txt
python HLAassoc.py -i input0.txt
python HLAassoc.py --file input0.txt --digits 4 --test chisq --model allelic --freq 0.05 --adjust FDR --print False --out hlaassoc.txt
Test all alleles with frequency higher than 0.001 and perform 10000 permutation test (it takes about 1 hour):
python HLAassoc.py --file input0.txt --perm 10000 --freq 0.001
Time used to perform 10000 permuatation using the example data.
| Test | Frequency | digit | perm | Wall time |
|---|---|---|---|---|
| chisq | 0.05 | 4 | 10000 | 4min 45s |
| fisher | 0.05 | 4 | 10000 | 1h 5min 32s |
| raw | 0.05 | 4 | 10000 | 4min 19s |
| score | 0.05 | 4 | 10000 | 4min 28s |
| logistic | 0.05 | 4 | 10000 | 23min 59s |
| linear | 0.05 | 4 | 10000 | 17min 21s |
| chisq | 0.001 | 4 | 10000 | 53min 35s |
| fisher | 0.001 | 4 | 10000 | 2h 49min 2s |
| raw | 0.001 | 4 | 10000 | 51min 4s |
| score | 0.001 | 4 | 10000 | 51min 16s |
| logistic | 0.001 | 4 | 10000 | 2h 14min 20s |
| linear | 0.05 | 4 | 10000 | 1h 33min 1s |
Note: Only simulated data with the same common alleles as the original data was used. The number of simulation usually is larger than the number perm, especially when lower frequency alleles were included. If there are only several alleles in cases and controls in the original data, these alleles may only occur in cases or controls in a simulated data.
Note: Order of options do NOT matter.
- Fisher's exact test
Fisher's exact test shares the same parameters with Pearson's chi-squared test. The following three commands are equivalent.
python HLAassoc.py --file input0.txt --test fisher
python HLAassoc.py -i input0.txt -t fisher
python HLAassoc.py --file input0.txt --digits 4 --test fisher --model allelic --freq 0.05 --adjust FDR --print False --out hlaassoc.txt
- Logistic regression
python HLAassoc.py -i input0.txt -t logistic
python HLAassoc.py --file input0.txt --test logistic
python HLAassoc.py --file input0.txt --digits 4 --test logistic --freq 0.05 --adjust FDR --print False --out hlaassoc.txt
Logistic regression with covariants: the first command uses all variants in covar.txt as covariants, the second command uses age and bmi as covariants.
python HLAassoc.py --file input0.txt --digits 4 --test logistic --freq 0.05 --adjust FDR --print False --out hlaassoc.txt --covar covar.txt
python HLAassoc.py --file input0.txt --digits 4 --test logistic --freq 0.05 --adjust FDR --print False --out hlaassoc.txt --covar covar.txt --covarname age,bmi
- Raw test
python HLAassoc.py -i input0.txt -t raw
python HLAassoc.py --file input0.txt --test raw
python HLAassoc.py --file input0.txt --digits 4 --test raw --freq 0.05 --print False --out hlaassoc.txt
Note: Raw test performs a Pearson's chi-squared test on the 2 x m contingency tables for each gene. m is the number of alleles in both cases and controls with frequency higher than the frequency threshold. Please be noted that when perform permutation test, the alleles used in the simulated data may different from that in the original data. For example, a rare allele can be in both cases and controls in the original data, but only found in cases or controls in a simulated data. When this is the situation, the permutation p value is meaningless.
- Score test
python HLAassoc.py -i input0.txt -t score
python HLAassoc.py --file input0.txt --test score
python HLAassoc.py --file input0.txt --digits 4 --test score --freq 0.05 --print False --out hlaassoc.txt
Note: The permutation p value is also meaningless when alleles used in the original data and simulated data are different.
6.2) Quantitative trait
- Linear regression
python HLAassoc.py -i input1.txt -t linear
python HLAassoc.py --file input1.txt --test linear
python HLAassoc.py --file input1.txt --digits 4 --test linear --freq 0.05 --adjust FDR --print False --out hlaassoc.txt
Linear regression with covariants: the first command uses all variants in covar.txt as covariants, the second command uses age and bmi as covariants.
python HLAassoc.py --file input1.txt --digits 4 --test linear --freq 0.05 --adjust FDR --print False --out hlaassoc.txt --covar covar.txt
python HLAassoc.py --file input1.txt --digits 4 --test linear --freq 0.05 --adjust FDR --print False --out hlaassoc.txt --covar covar.txt --covarname age,bmi