CONICS
CONICS: COpy-Number analysis In single-Cell RNA-Sequencing
CONICS works with either full transcript (e.g. Fluidigm C1) or 5'/3' tagged (e.g. 10X Genomics) data!
The CONICS paper has been accepted for publication in Bioinformatics. Check it out here !
Table of contents
- CONICSmat - Identifying CNVs from scRNA-seq with only a count table (R tutorial)
- Identifying CNVs from scRNA-seq using aligned reads and a control dataset (advanced users)
- Integrating the minor-allele frequencies of point mutations (advanced users)
- Phylogenetic tree contruction
- Intra-clone co-expression networks
- Assessing the correlation of CNV status with single-cell expression
- False discovery rate estimation: Cross validation
- False discovery rate estimation: Empirical testing
CONICSmat - Identifying CNVs from scRNA-seq using a count table
CONICSmat is an R package that can be used to identify CNVs in single cell RNA-seq data from a gene expression table, without the need of an explicit normal control dataset. CONICSmat works with either full transcript (e.g. Fluidigm C1) or 5'/3' tagged (e.g. 10X Genomics) data. A tutorial on how to use CONICSmat, and a Smart-Seq2 dataset, can be found on the CONICSmat Wiki page [CLICK here].
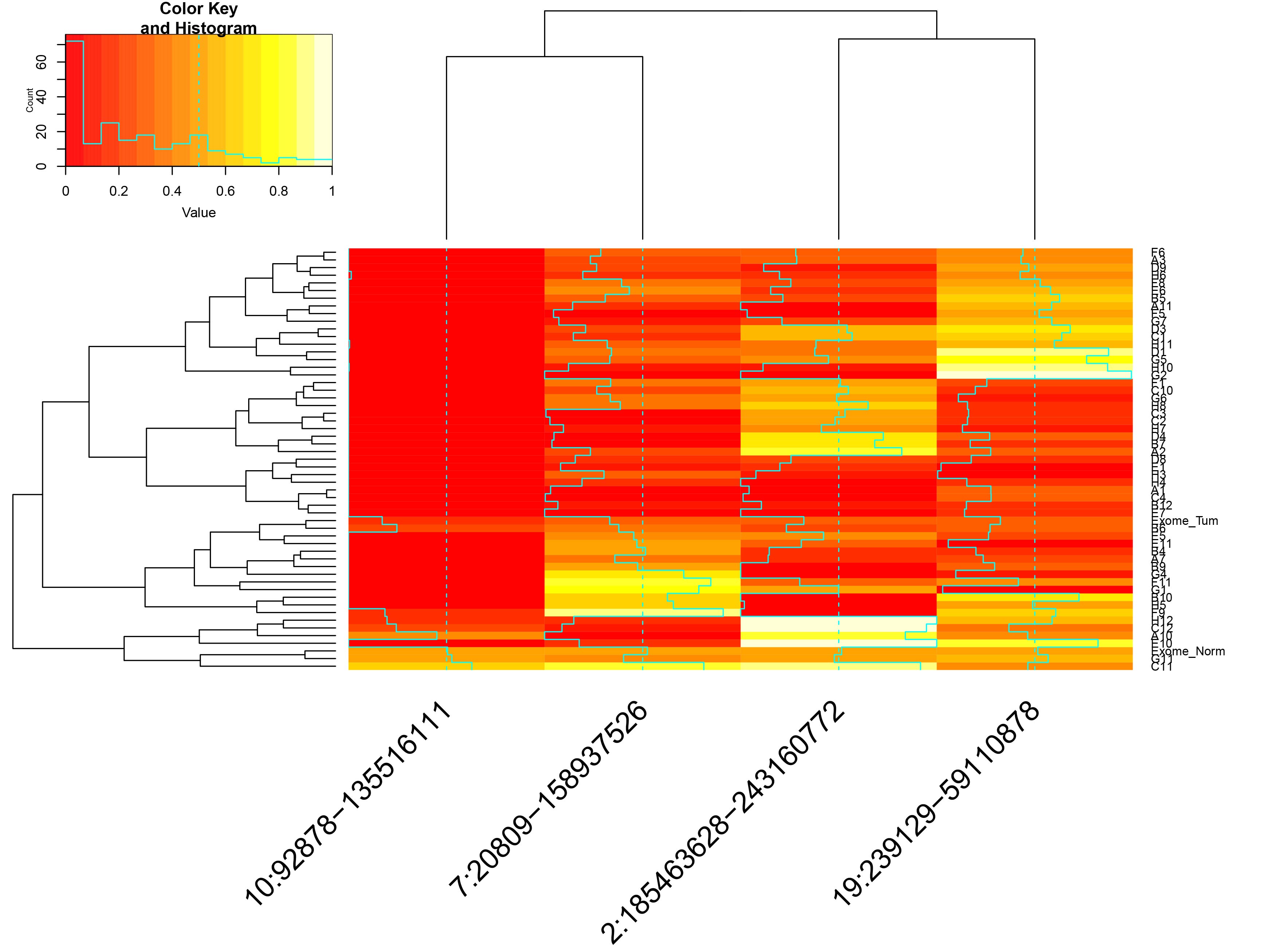
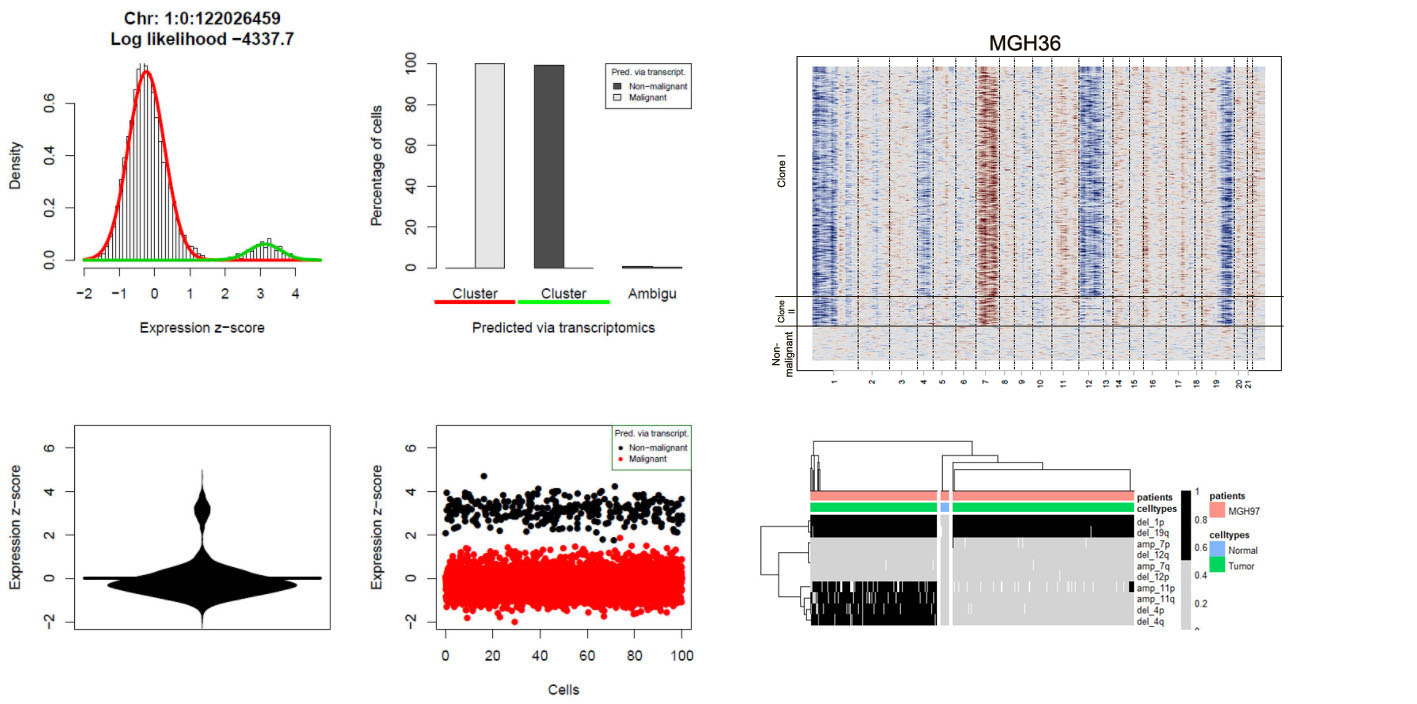 Visualizations of scRNA-seq data from Oligodendroglioma (Tirosh et al., 2016) generated with CONICSmat.
Visualizations of scRNA-seq data from Oligodendroglioma (Tirosh et al., 2016) generated with CONICSmat.
CONICS - Identifying CNVs from scRNA-seq with alignment files
Requirements
- Python and Perl
- beanplot
- samtools
- bedtools IMPORTANT: Bedtools >2.2.5 is needed in order to correctly calculate the coverage using CONICS.
- Two directories, the first containing the aligned scRNA-seq data to be classified by CNV status, and a second, containing aligned scRNA-seq data to be used as a control.
- A file containing the genomic coordinates of the CNVs in BED format.
Config file
Adjust CONICS.cfg to customize the following:
- Path to python/samtools/bedtools/Rscript
- Thresholds for mapping-quality and read-count
- FDR for CNV calling
Test data
A set of tumor cells from three glioblastoma patients and a normal brain control, as well as a file with genomic coordinate of large-scale CNVs are available here.
Running
bash run_CONICS.sh [directory for tumor] [directory for normal] [.bed file for CNV segments] [base name]
-
[directory for tumor]: path to directory containing aligned bam files to be tested. Example glioblastoma data, used in the manuscript, can be obtained here.
-
[directory for normal]: path to directory containing aligned bam files to be used as a control. Example nonmalignant brain data, used in the manuscript, can be obtained here was used as an examples for the journal
-
[BED file for CNV segments]: tab-delimited bed file of CNV segments to be quantified.
[chromosome] [start] [end] [chromosome:start:end:CNV]Note: the 4th column of the file must have the exact format shown here:(Amp: amplification, Del: deletion)
7 19533 157408385 7:19533:157408385:Amp
9 19116859 32405639 9:19116859:32405639:Del
- [base name] : base name for output directory
Output
All output files will be located in the directory output[base name]_.
- incidenceMatrix.csv: matrix of presence/absence for all CNVs, in individual cells
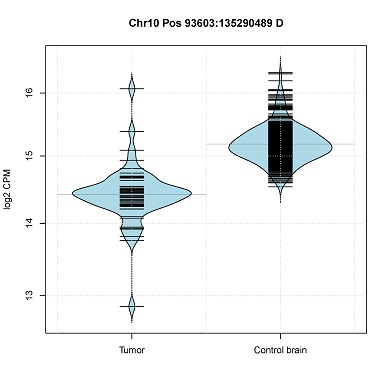
- Read-count distribution in CNV segments. (violin plot)
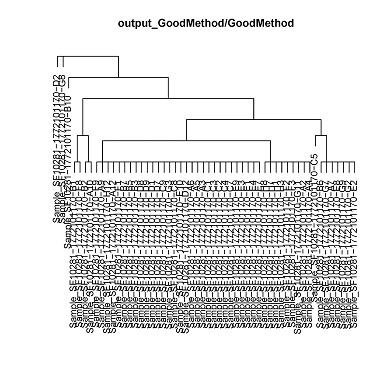
- Hierarchical clustering of the single cells by CNV status.
Integrating estimates of point-mutation minor-allele frequencies
Regions of copy-number alteration will show a drop in the frequency of reads quantifying the minor allele. Averaged over large regions of copy-number alteration, this provides an additional metric to increase confidence in single-cell CNV-calls.
Requirements
- Python and R
- bam-readcount
- gplots and ggplot2
- One directory containing the aligned tumor scRNA-seq data to be classified
- Two variant VCF files from exome-seq of (blood) control and tumor tissue, eg generated with the GATK toolkit.
- A file containing the genomic coordinates of the CNVs in BED format.
Config file
Adjust CONICS.cfg to customize the following:
- Path to python/bam-readcount/Rscript
- Path to genome which reads were aligned to (FASTA format)
Running
bash run_BAf_analysis.sh [directory for tumor] [VCF file for normal exome-seq] [VCF file for tumor exome-seq] [BED file for CNV segments] [base name]
-
[directory for tumor]: path to directory containing aligned bam files to be tested. Example glioblastoma data, used in the manuscript, can be obtained here.
-
[VCF file for normal exome-seq]: Vcf file containing mutations for a control exome-seq, e.g. from blood of the patient. This file can be generated with tools like GATK toolkit.
-
[VCF file for tumor exome-seq]: Vcf file containing mutations detected in exome-seq of the tumor. This file can be generated with tools like GATK toolkit.
-
[BED file for CNV segments]: tab-delimited bed file of CNV segments to be quantified.
-
[base name] : base name for output directory
Output
All output files will be located in the directory output[base name]_
- _germline-snvs.bed: BED file containing position and BAFs from Exome-seq, generated in step 1.
- _af.txt: TAB separated table containing the counts for the A allele at each locus in each cell, generated in step 2
- _bf.txt: TAB separated table containing the counts for the B allele at each locus in each cell, generated in step 2
- baf_hist.pdf Hierarchical clustering of the average B allele frequency in each of the loci altered by copy number for each cell, generated in step 3
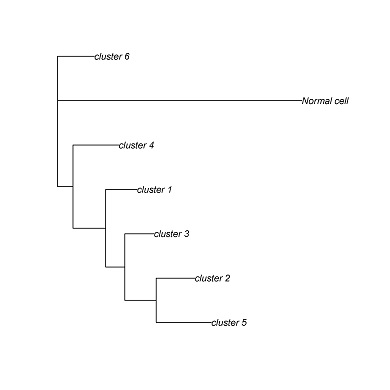
Phylogenetic tree contruction
CONICS can generate a phylogenetic tree from the CNV incidence matrix, using the Fitch-Margoliash algorithm. Other phylogenetic reconstruction algorithms can be applied, using the incidence matrix as a starting point.
Requirements
Config file
Adjust Tree.cfg to change the following.
- Path to Rscript
- Path to Rphylip
Running
- Before running, set the path to Phylip in Tree.cfg file.
bash run_Tree.sh [CNV presence/absence matrix][number of genotypes] [base name for output file]
- [CNV presence/absence matrix]: .incidenceMatrix.csv files.
- [number of genotypes]: the number of genotypes to model
- [base name] : base name for output directory
Output
cluster.pdf (phylogenetic trees) and cluster.txt will be generated in the output directory. Each leaf corresponds to a clusters of cells with a common genotype. Cluster assignments for each cell will be in cluster.txt.
cluster_1 D12,E10,F9,G3,A12,C8,C9,A3,A5,A6,C3,C2,C1,C7,H12,C4,D8,D9,A9,E4,E7,E3,F1,E1,B5,B7,E9,B3,D7,D1
cluster_2 E8,G7,G9,A7,G2,B6,E2
cluster_3 H3,A2,A4,H8,G11,F2,F3,H1,H7
cluster_4 A10,B2
cluster_5 C5
cluster_6 F8,B1
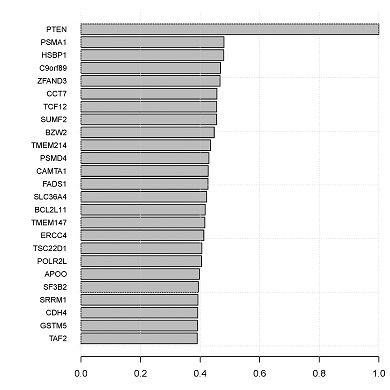
Intra-clone co-expression networks
CONICS can construct the local co-expression network of a given gene, based on correlations across single cells.
Requirements
Config file
Adjust CorrelationNetwork.cfg to configure the following:
- Path to Rscript
- ncore: Number of cores (default: 12)
- cor_threshold: Starting threshold to construct the co-expression network (default: 0.9)
- min_neighbours: How many direct neighbours of gene of interest should be analyzed (default: 20)
- minRawReads: How many raw reads should map to a gene for it to be included (default: 100)
- percentCellsExpressing: Percentage (0.15 =15%) of cells expressing a gene for it to be included (default: 0.15)
- minGenesExpr: How many genes should be expressed in a cell for it to be included (default: 800)
- depth: How deep should the gene analysis search. (2=only direct neighbor genes would be considered) (default: 2)
Running
bash run_CorrelationNetwork.sh [input matrix] [centered gene] [base name]
- [input matrix]: tab-delimited file of read counts for each gene (rows), for each cell (columns).
- [centered gene]: a target gene of which neighbor genes are analyzed.
- [base name] : base name for output directory
Output
All the output files will be located in output.
- [correlstion_threshold]_[gene_name].txt : co-expression network
- [gene_name]corMat.rd: Rdata containing the adjusted correlation matrix
- topCorrelations.pdf: bar graph of top correlations.
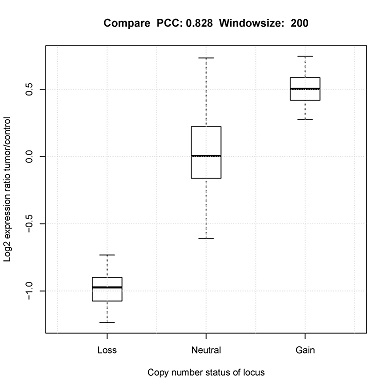
Assessing the correlation of CNV status with single-cell gene-expression
Requirements
Config file
Adjust CompareExomeSeq_vs_ScRNAseq.cfg to set the following:
- Path to Rscript
- window size for assessing CNV status
Running
bash run_compareExomeSeq_vs_ScRNAseq.sh [matrix for read counts] [base name for output file]
-
[matrix for read counts]: tab-delimited file of the number of mapped reads to each gene in the DNA sequencing and in scRNA-seq. Genes on each chromosome should be ordered by their chromosomal position.
[gene] [chromosome] [start] [#read in DNA-seq(normal)] [#read in DNA-seq(tumor)] [#read in scRNA-seq(normal)] [#read in scRNA-seq(tumor)]- example
DDX11L1 1 11874 538 199 5 0
WASH7P 1 14362 4263 6541 223 45
- [base name] : base name for output directory
Output
Compare[window_size].pdf_ (Box plot) will be generated in the output directory.
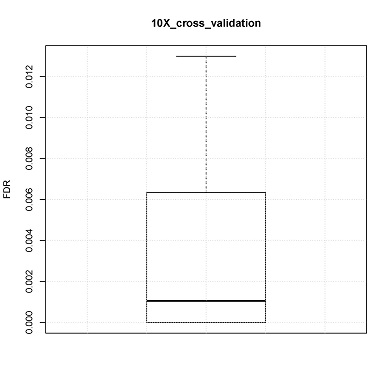
False discovery rate estimation: Cross validation
CONICS can estimate false discovery rate via 10-fold cross-validation, using the user-supplied control scRNA-seq dataset. For example, in the manuscript cross validation was performed using normal brain controls.
Requirements
Config file
Adjust 10X_cross_validation.cfg to set the following:
- Paths to python/samtools/bedtools/Rscript
- Thresholds for mapping-quality and read-count.
- FDR for CNV calling
Running
bash run_10X_cross_validation.sh [directory for control scRNA-seq] [.bed file containing CNV segments] [base name]
-
[directory for test]: path to directory containing the aligned BAM files of the scRNA-seq control data.
-
[.bed file for CNV segments], [base name] : same as described in run_CONICS.sh ;
Output
Box plot of 10 FDRs resulting from each pooled sample would be generated (boxplot.pdf) in the output directory.
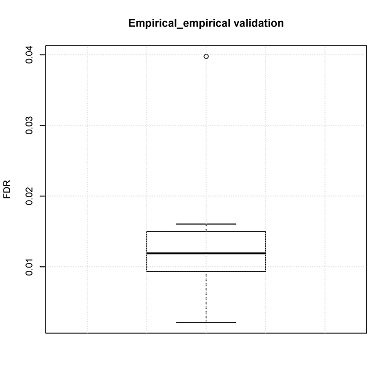
False discovery rate estimation: Empirical testing
FDRs can also be estimated by empirical testing. In the manuscript, the number of false positive CNV calls was calculated using a non-malignant fetal brain dataset. These data are independent from the training set
Requirements
Config file
Adjust Empirical_validation.cfg to change the following:
- Paths to python/samtools/bedtools/Rscript
- Thresholds for mapping-quality and read count
- FDR for CNV calling
Running
bash run_empirical_validation.sh [directory for train] [directory for test] [.bed files for CNV segments] [base name]
-
[directory for train]: path to directory containing aligned bam files of scRNA-seq data used as a control to call CNVs
-
[directory for test]: path to directory containing aligned bam files of scRNA-seq data known not to have CNVs, used as a gold standard.
-
[BED file for CNV segments] : same as described in run_CONICS.sh
Output
Box plot of FDRs will be generated (boxplot.pdf) in the output directory.