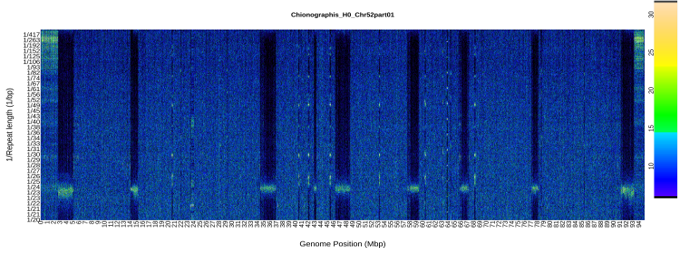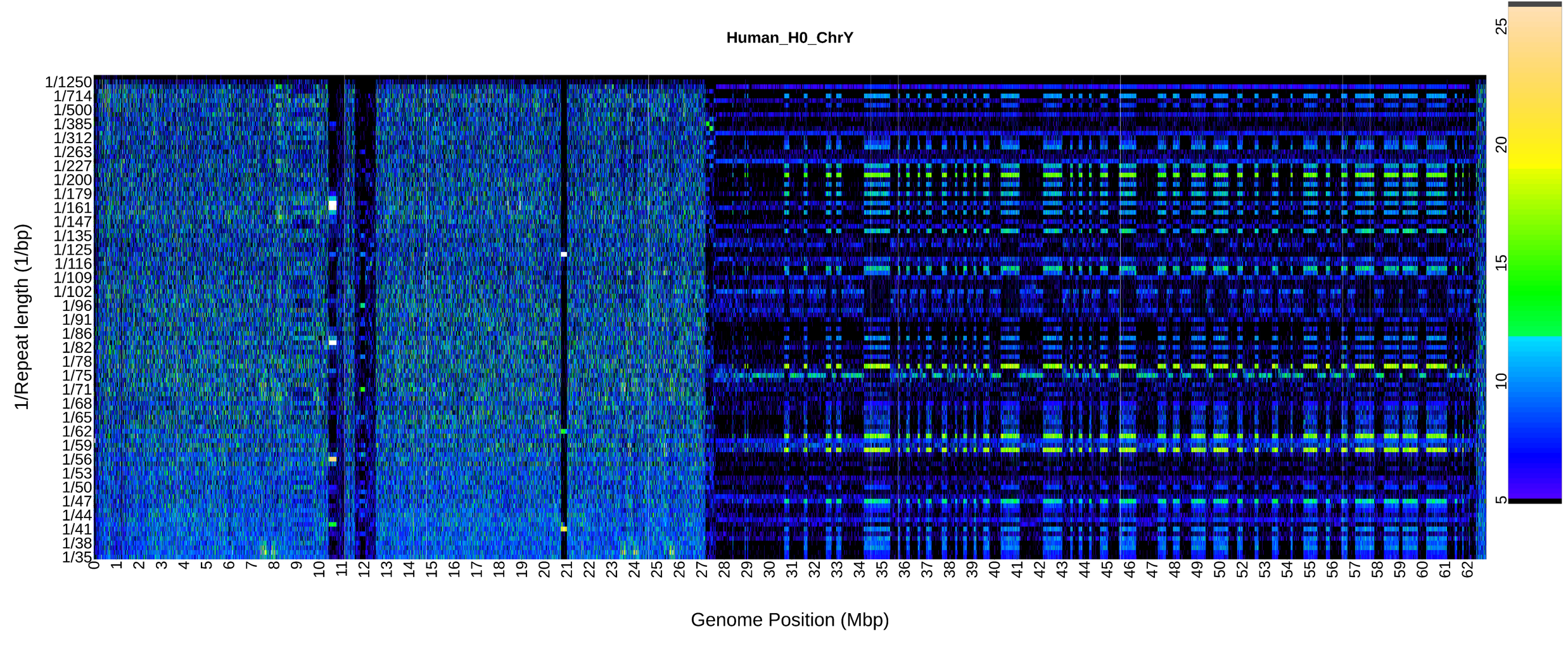
An R package to visualize chromosome scale repeat patterns and predict centromere locations.
Report Bug
Table of Contents
RepeatOBserver is an R package that can be run on any chromosome scale reference genome assembly (e.g. fasta file). RepeatOBserver returns many plots describing the tandem repeats and clusters of transposons found across each chromosome. Based on the repeat patterns, RepeatOBserver also returns a predicted centromere location for each chromosome based on the repeat diversity across that chromosome.
You can learn more about the interpretations of the plots in our manuscript here: https://www.biorxiv.org/content/10.1101/2023.12.30.573697v1
The following software are need to run the automatic RepeatOBserver script:
- seqkit/2.3.1 : https://bioinf.shenwei.me/seqkit/
- r/4.1.2 : https://cran.r-project.org/bin/windows/base/old/
- (optional to see isochores) emboss/6.6.0 : https://emboss.sourceforge.net/download/
Newer versions of these software may work but the program has not yet been tested throughly in them. If you are unable to install any of the programs above you can run the RepeatOBserver code in R but the automated bash script will not work for you (see Troubleshooting at the end of this page for details on how to run the code without this script).
Example software installation (using Compute Canada modules):
module load seqkit/2.3.1
module load StdEnv/2020
module load emboss/6.6.0
module load r/4.1.2To install the R package "RepeatOBserverV1", you will first need to install the package devtools in your version of R.
install.packages("devtools")
library(devtools)
install_github("celphin/RepeatOBserverV1") #to install the package
# Select 1:All to install all the required packages
library(RepeatOBserverV1) # to load the package
Feb 20th, 2024 : The older Setup_Run_Repeats_preFeb20.sh may still work but it is ideal to rerun the program from scratch (i.e. download the new script, reinstall R library and delete old folders). Changes include:
- Ablity to run longer chromosomes in 400Mbp parts
- An easier to use CGwalk and transform option
- A changing in the output file structure and matching output documentation below
- A list of the original chromosome names and what they have been renamed in the program
- Summary plots showing all the chromosomes in one plot
Note that you should not get any different results but restarting the program with the old script may no longer work.
Example new plot:
Download a copy of the Setup_Run_Repeats.sh script from this github repo into the directory that you want to run the code in.
wget https://raw.githubusercontent.com/celphin/RepeatOBserverV1/main/Setup_Run_Repeats.shMake sure the script is executable and setup to run on unix.
chmod +x Setup_Run_Repeats.sh
dos2unix Setup_Run_Repeats.shMove your chromosome scale fasta file (needs to contain more than one chromosome, ie. a genome) into a directory that you want to run RepeatOBserverV1 in. Make sure to unzip/gunzip the file. In this directory, with your desired reference genome, you can run the default RepeatOBserverV1 commands automatically with the following command:
sh Setup_Run_Repeats.sh -i SpeciesName -f Reference_Genome.fasta -h H0 -c c -m m -g FALSENecessary parameters:
| Parameter | Usage | Example Input |
|---|---|---|
| -i | Species Name | Fagopyrum (cannot contain an _ or space) |
| -f | Reference genome fasta file | Fagopyrum_Main.fasta |
| -h | Haplotype (string) | H0 (cannot contain an _ or space) |
| -c | cpus available (any integer value) | 20 |
| -m | memory available (MB) | 128000 |
| -g | FALSE to run for AT DNAwalk or TRUE to run for CG DNAwalk | FALSE |
If you require an allocation to get enough memory or cpu (125G for 15 CPU is best) on your server, here is a slurm template to follow:
cat << EOF > SPP_repeats.sh
#!/bin/bash
#SBATCH --account=<your-account>
#SBATCH --time=5:00:00
#SBATCH --ntasks=1
#SBATCH --cpus-per-task=15
#SBATCH --mem=128000M
module load StdEnv/2020
module load seqkit/2.3.1
module load emboss/6.6.0
module load r/4.3.1
srun Setup_Run_Repeats.sh -i SpeciesName -f Reference_Genome.fasta -h H0 -c c -m m -g FALSE
EOF
sbatch SPP_repeats.shSome example/test code can be found here.
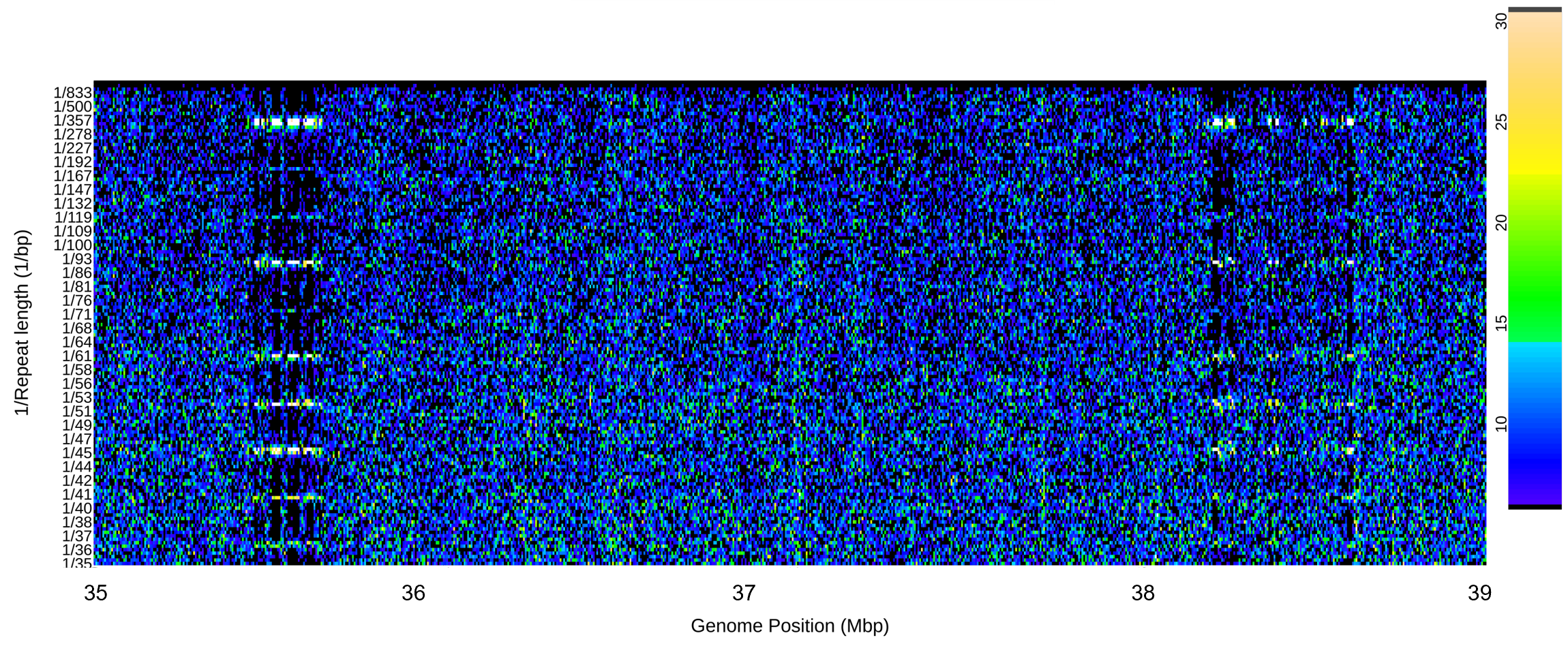
After running the Wine genome you should get the following plot for chromosome 7 repeat lengths 15 to 35 bp.
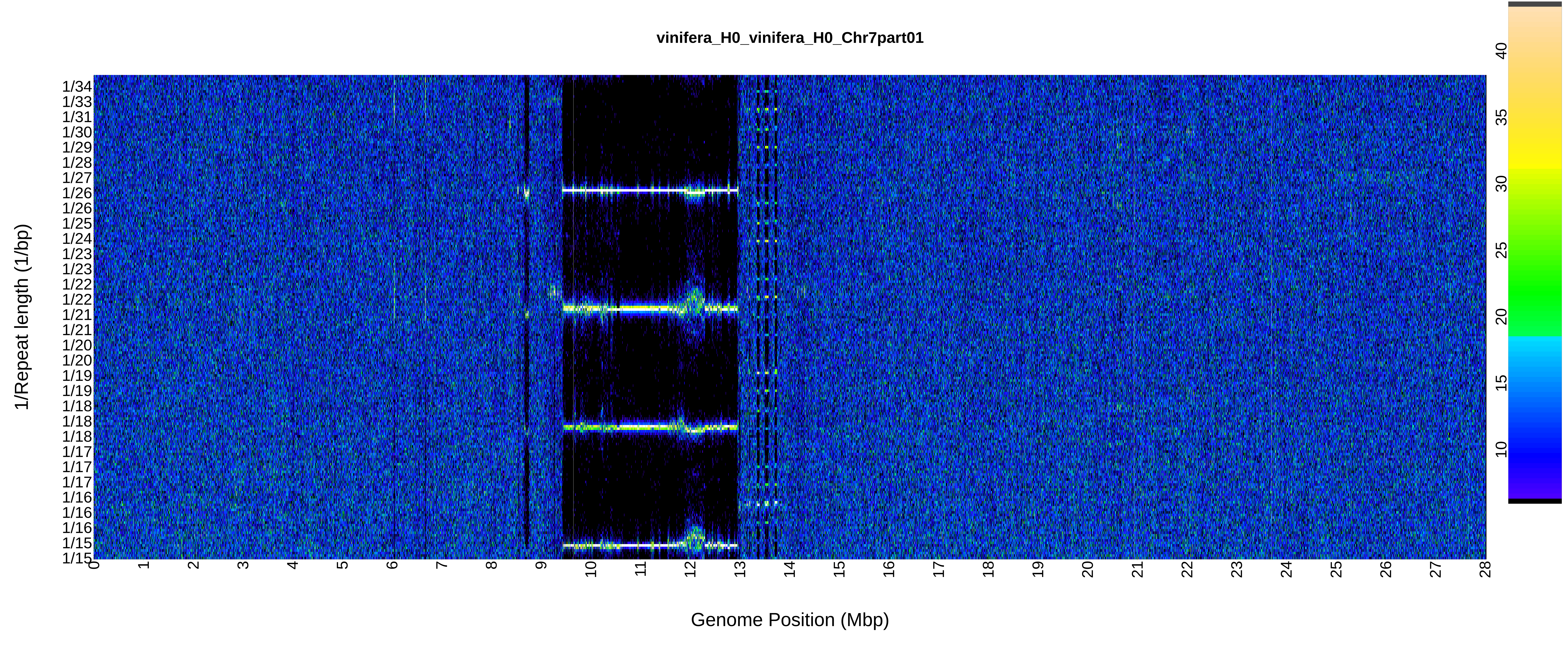
Summary plots and output files can be found in:
cd <your-starting-directory>/output_chromosomes/Species_Haplotype/Summary_output
Note: chromosomes are named differently than in the original fasta file and you can find the new names in chromosome_renaming.txt
You can use the 'tree' command in the folder above to see all subfolders and files described below. Folders that are missing from the list above did not finish. The program removes any scaffolds or chromosomes less than 5Mbp. You can try restarting the script with the exact same submission as before and it will start where it left off if it did not finish due to time restraints.
Output folders and summary files that you should find in the directory above, if the whole program worked:
| Main folders | Description (more details below) |
|---|---|
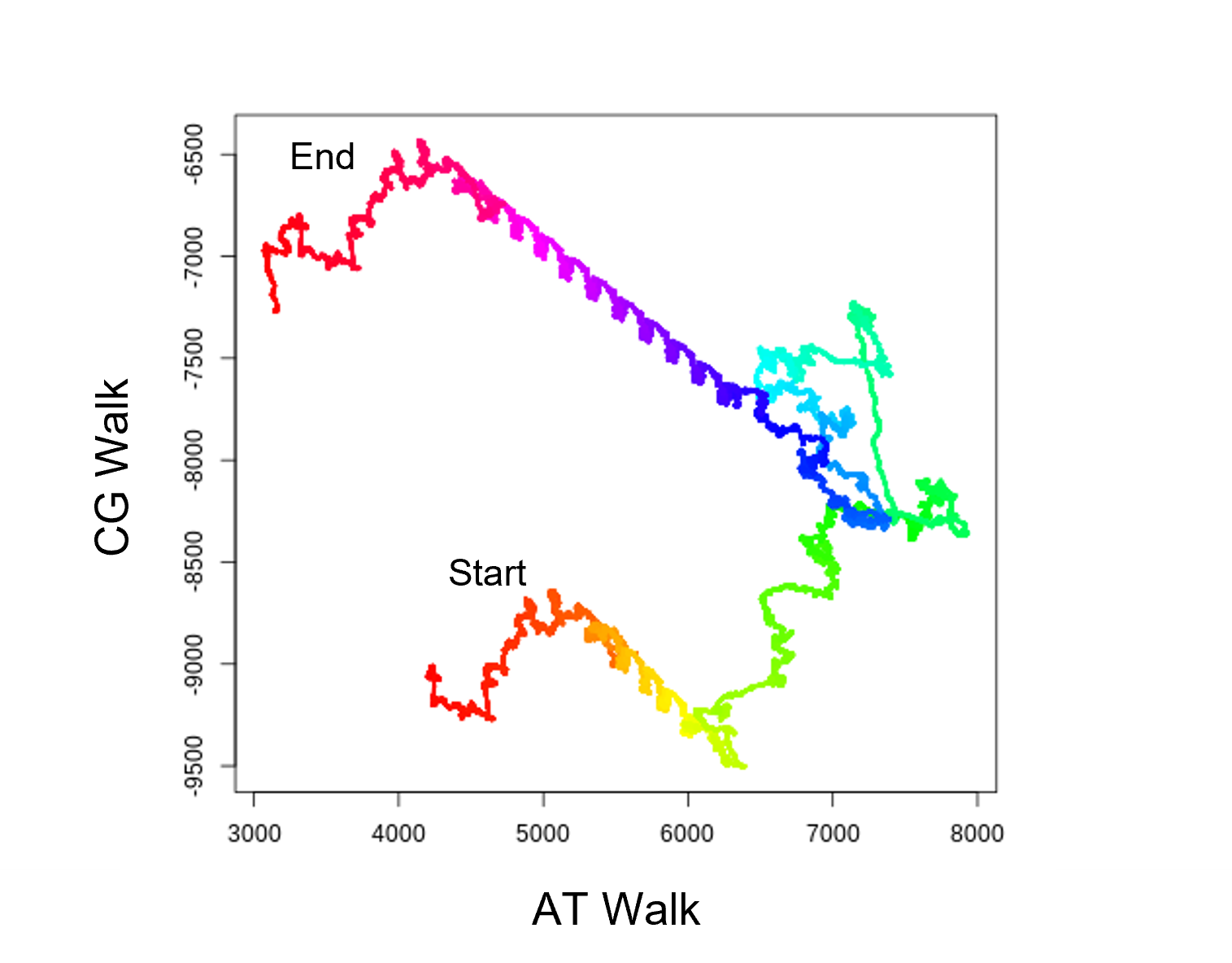
| DNAwalks | 1D and 2D DNAwalks |
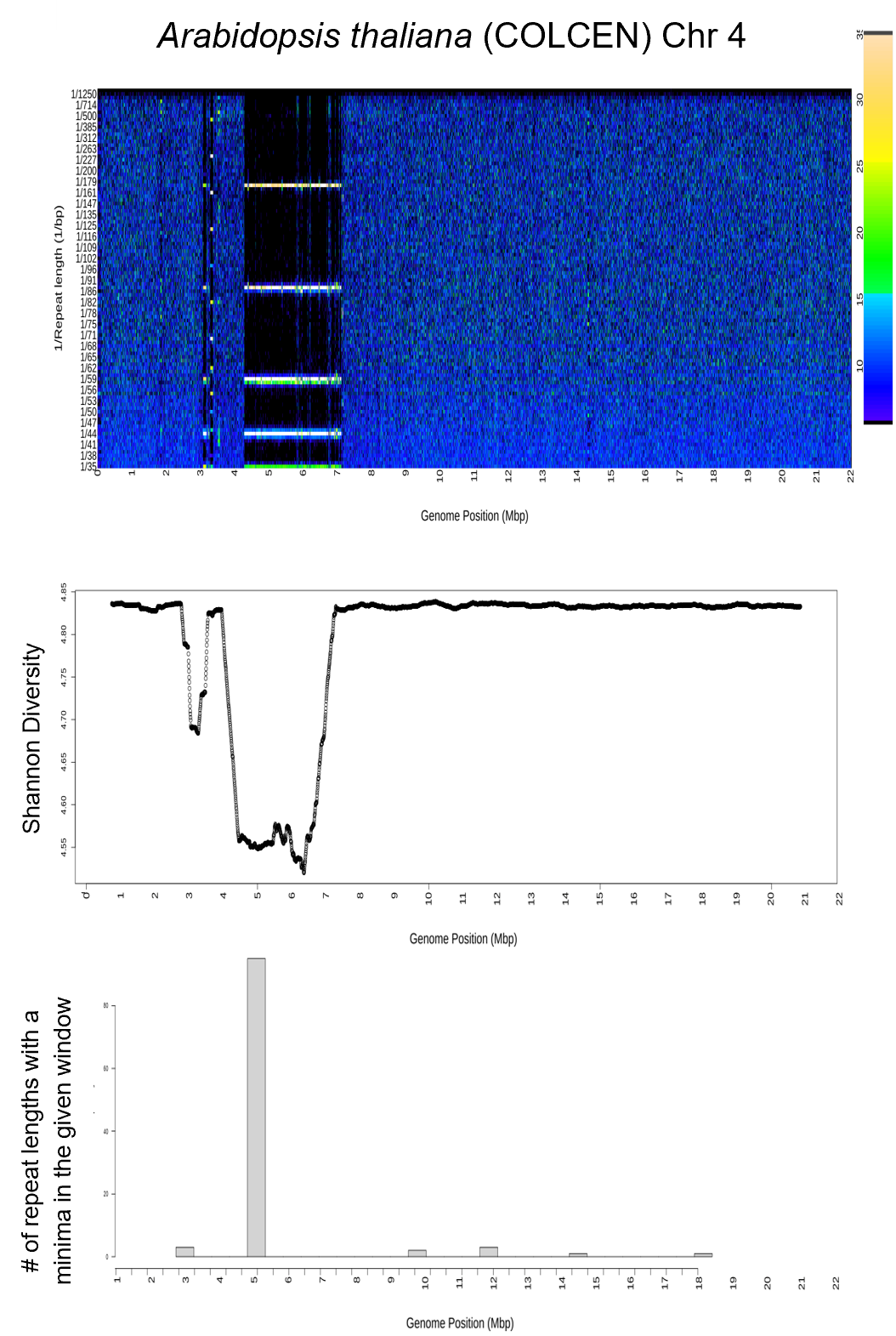
| histograms | histogram centromere predictions and plots |
| output_data | Raw data files including Shannon diversity, DNAwalks, Fourier transforms |
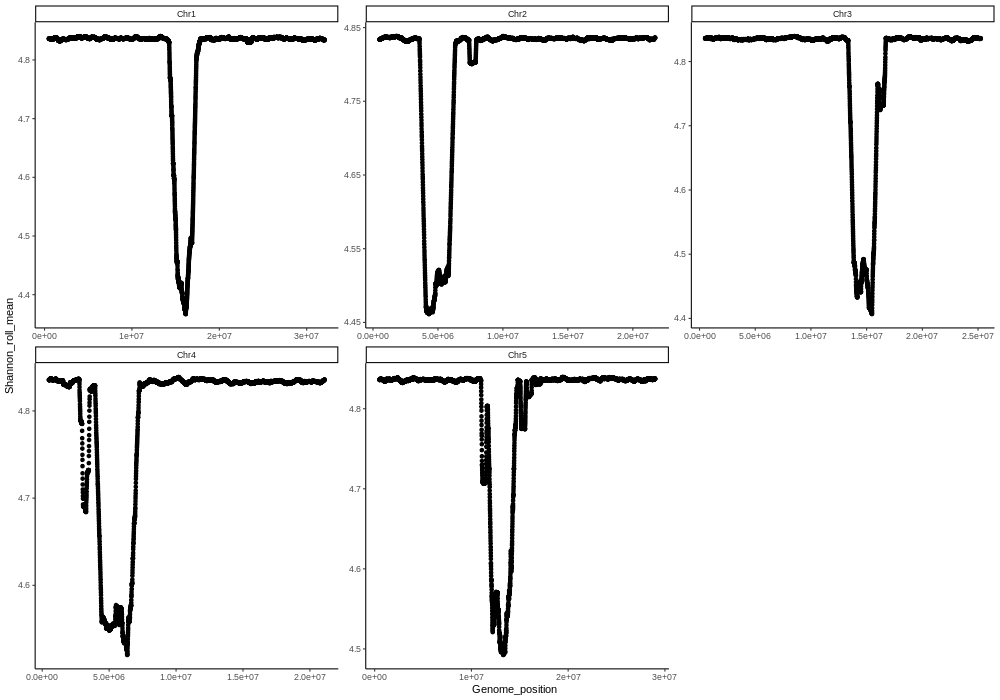
| Shannon_div | Shannon diversity plots for each chromosome |
| spectra | Heat maps of the Fourier transform output |
| isochores | CG isochores plot made with the EMBOSS program, useful to see if centromere positions are associated with isochores |
| Summary files | Description |
|---|---|
| chromosome_renaming.txt | New chromosome names assigned to each chromosome in the program |
| Species_Haplotype_Histograms.png | All chromosomes histograms plotted in one figure |
| Species_Haplotype_Shannon_div.png | All chromosomes Shannon_div plotted in one figure |
| Species_Haplotype_rolling_mean_500Kbp_Shannon_div.png | All chromosomes Shannon_div in 500kbp rolling windows plotted in one figure |
| Folder/file name | Description | Example file |
|---|---|---|
| 1D | 1D CG and AT DNAwalks, rainbow colours change every 10Kbp | Species_Haplotype_Chr1_DNAwalk1D_AT_total.png |
| 2D | 2D DNAwalks, the 1D walks plotted against each other | Species_Haplotype_Chr1_DNAwalk2D_total.png |
| Folder/file name | Description |
|---|---|
| Centromere_histograms_summary.txt | The predicted centromere positions for every chromosome based on the histogram output |
| Species_Haplotype_Chr1_histogram_....png | Histogram plots showing counts of where in the genome each repeat length minimized |
| Folder/file name | Description |
|---|---|
| Species_Haplotype_Chr1_Histogram_input_....txt | The Fourier window that each repeat length minimized in can be used to build the histograms |
| Species_Haplotype_Chr1_Shannon_div.txt | The raw Shannon diversity data for each 5kbp window in the genome |
| Total_dnawalk_every50_Species_Haplotype_Chr1.txt | The raw DNAwalk data sampled every 50bp (see chromosome folders for the complete walk) |
| Total_Species_Haplotype_Chr1_All_spec_merged.txt | The Fourier transform output merged for up to 400Mbp (chromosomes >400Mbp will be in parts) |
| Folder/file name | Description | Example file |
|---|---|---|
| Main folder | The predicted centromere predictions using Shannon diversity with varying rolling mean window sizes, columns are: # of windows averaged, Centromere_prediction, Total_chr_length, Spp, Chr | Centromere_summary_Shannon_1000.txt |
| Shannon_div_5kbp | Raw Shannon diversity values (no averaging) for each 5kbp Fourier window | Species_Haplotype_Chr1_Shannon_plot_norm.png |
| Shannon_div_500kbp | Shannon diversity values averaged with rolling window across 100 windows (500kbp region) | Species_Haplotype_Chr1_roll_mean_Shannon_100.png |
| Shannon_div_1.25Mbp | Shannon diversity values averaged with rolling window across 250 windows (1.25Mbp region) | Species_Haplotype_Chr1_roll_mean_Shannon_250.png |
| Shannon_div_2.5Mbp | Shannon diversity values averaged with rolling window across 500 windows (2.5Mbp region) | Species_Haplotype_Chr1_roll_mean_Shannon_500.png |
| Shannon_div_5Mbp | Shannon diversity values averaged with rolling window across 1000 windows (5Mbp region) | Species_Haplotype_Chr1_roll_mean_Shannon_1000.png |
| Shannon_div_window | Rolling mean of Shannon diversity values with window size determined by genome size | Species_Haplotype_Chr1_Shannon_div_window210.png |
| Folder/file name | Description | Example file |
|---|---|---|
| spectra_total_merged | Heat maps of the Fourier transforms of the whole chromosomes (up to 400 Mbp) for long repeat lengths 35-2000 bp | Species_Haplotype_Chr1_All_spec_bp35_2000seq1_6510TRUE.png |
| spectra_parts_2-8 | Heat maps of the Fourier transforms of 100 Mbp chromosome parts for short repeat lengths 2-8 bp | All_spec1_Species_Haplotype_Chr1part01_bp15_35seq2501_32542501TRUE.png |
| spectra_parts_15-35 | Heat maps of the Fourier transforms of 100 Mbp chromosome parts for mid repeat lengths 15-35 bp | All_spec1_Species_Haplotype_Chr1part01_bp2_8seq2501_32542501TRUE.png |
| spectra_parts_35-2000 | Heat maps of the Fourier transforms of 100 Mbp chromosome parts for long repeat lengths 35-2000 bp | All_spec1_Species_Haplotype_Chr1part01_bp35_2000seq2501_32542501TRUE.png |
If you have many or large gaps in your genome, the centromere predictions using Shannon diversity windows may not be accurate. You can check for gaps or unusual characters in your genome with the following commands:
# use seqkit to split into 60bp lines
module load seqkit/2.3.1
seqkit seq -w 60 genome.fasta > genome.fasta
# search for any non-nucleotide characters
grep [^ATCGatcg] genome.fasta
# to remove gaps from your genome - replace the n below with the gap character
# note that this is not a longer good idea since you will lose the information about where your gaps are in the genome
sed 's/n//g' genome.fasta > genome_rmgaps.fasta
Using an EMBOSS function (equicktandem), we can find the tandem repeat sequences quickly once the position has been roughly located with RepeatOBserver.
https://bar.utoronto.ca/cgi-bin/emboss/help/equicktandem
Note that very imperfect repeats or longer may not show up but short repeats are easy to detect and compare using the following script.
To run this script EMBOSS6.6.0 and some version of seqkit is required.
module load nixpkgs/16.09 gcc/7.3.0 emboss/6.6.0
module load StdEnv/2020 seqkit/2.3.1First download the script:
wget https://raw.githubusercontent.com/celphin/RepeatOBserverV1/main/repeat_seq_finder.sh
chmod +x repeat_seq_finder.sh
dos2unix repeat_seq_finder.shScript inputs (in order with an example):
- Direct the script to your chromosome files: "/home/celphin/scratch/repeats/auto_script/input_chromosomes/NerLuet_H0-AT/chromosome_files"
- Choose the chromosome fasta file that you want to look at the repeat in: "NerLuet_H0-AT_Chr1part01.fasta"
- Define the bp range that you want to search in (this is selected manually using the Fourier heatmaps or filtering for low Shannon diversity values (start must be 60 or more)): 13200000 13500000
- Define the upper repeat length you are looking for: 4 (will work for repeats up to 600bp long, it is better to choose a bit larger value from the heatmap if uncertain)
- Define the number of bp per line in your fasta file: 60 (default for RepeatOBserverV1 - change only if you adjusted the chromosome files afterwards)
- Define the threshold for calling a tandem repeat (note that longer repeats get lower scores generally so can lower the threshold, 500 reasonable for small and 200 for large repeats)
Here is an example of running it on an algae repeat visualized using RepeatOBserver
# Example short run
../repeat_seq_finder.sh "/home/celphin/scratch/repeats/auto_script/input_chromosomes/NerLuet_H0-AT/chromosome_files" \
"NerLuet_H0-AT_Chr1part01.fasta" 9600000 9700000 3 60 500
# cta
# tactactactactactactac
#Example of a longer repeat
./repeat_seq_finder.sh "/home/celphin/scratch/repeats/auto_script/input_chromosomes/NerLuet_H0-AT/chromosome_files" \
"NerLuet_H0-AT_Chr1part01.fasta" 10000000 10500000 90 60 500
#cccgcccacgtgatgctgctgttttctcccgctttgacgaggttatagagggtatgatc gtacggggtctggtactgttgtgccccct
#ccctctcccatgatactgcagcactttcccgtttga cgggaaggtccagggtatgatcctgcggggtctggtactgttgtgccccct
Pre-print: Elphinstone, C., Elphinstone, R., Todesco, M., & Rieseberg, L. (2023). RepeatOBserver: Tandem repeat visualization and centromere detection (p. 2023.12.30.573697). bioRxiv. https://doi.org/10.1101/2023.12.30.573697
If you have questions, suggestions or comments please contact:
Cassandra Elphinstone - cassandra (dot) elphinstone (at) gmail (dot) com
Or post here: https://github.com/celphin/RepeatOBserverV1/discussions
After the initial Fourier transform has been run, there are many functions to explore and visualize the repeat patterns (described below). The default versions of these functions should automatically run in the script above. More specific plots can be made following the instructions below.
Use the run_summary_hist and run_diversity_plots functions:
# in R
library(RepeatOBserverV1)
inpath="~/scratch/repeats/input_chromosomes/Arab_COLCEN/chromosome_files/"
fname= "COLCEN_H0"
outpath="~/scratch/repeats/output_chromosomes"
x_cpu=1
pflag=FALSE
writeflag=FALSE
plotflag=FALSE
nam_list0 <- list.files(inpath)
nam_list1 <- tools::file_path_sans_ext(nam_list0)
nam_list1
nam_list2 <- stringr::str_split(nam_list1, "_", simplify =TRUE)
nam_list3 <- stringr::str_split(nam_list2[,3], "part", simplify =TRUE)
chr_list <- nam_list3[,1]
chr_list
# run chromosomes on different cpu
uni_chr_list <- unique(chr_list)
uni_chr_list
for (chromosome in uni_chr_list){
run_summary_hist(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
run_diversity_plots(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
}
Move summary information and plots for all the chromosomes into one folder. These commands should be run on a bash terminal. If these files cannot be found
#!/bin/bash
# set the SPP_Hap variable
SPP_Hap= <Yourspp_haplotype>
# change to the output directory path for your data - note that is may need to be changed in all that follows
cd ~/scratch/repeats/output_chromosomes/${SPP_Hap}/
mkdir Summary_output; cd Summary_output
cd ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Summary_output
#-----------
# copy over the Fourier heatmaps for various ranges of repeat lengths for each 100Mbp part
mkdir spectra_parts_35-2000
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/largeimages.png/All_spec1_*_Chr*_bp35_2000seq2501_*TRUE.png ./spectra_parts_35-2000
mkdir spectra_parts_15-35
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/largeimages.png/All_spec1_*_Chr*_bp15_35seq2501_*TRUE.png ./spectra_parts_15-35
mkdir spectra_parts_2-8
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/largeimages.png/All_spec1_*_Chr*_bp2_8seq2501_*TRUE.png ./spectra_parts_2-8
#------------------------------
# copy over the merged Fourier heatmaps, histogram plots and DNAwalks for the whole chromosomes
mkdir spectra_total_merged
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/*All_spec_bp35_2000*.png ./spectra_total_merged
mkdir histograms
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/histograms/*POWER_SUM*s_0.5std_1*.pdf ./histograms/
mkdir DNAwalks
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/*DNAwalk*.png ./DNAwalks
#----------
# copy Shannon diversity plots
mkdir Shannon_div
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/${SPP_Hap}_Chr*_Shannon_plot_norm.png ./Shannon_div
mkdir Shannon_div_100
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/*roll_mean_Shannon_100.png ./Shannon_div_100
mkdir Shannon_div_1000
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/*roll_mean_Shannon_1000.png ./Shannon_div_1000
mkdir Shannon_div_250
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/*roll_mean_Shannon_250.png ./Shannon_div_250
mkdir Shannon_div_500
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/*roll_mean_Shannon_500.png ./Shannon_div_500
mkdir Shannon_div_window
cp -v -u ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/*Shannon_div_window*.png ./Shannon_div_window
#-------------
# summarize the centromere results
rm Centromere_summary.txt
cat ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/histograms/Centromere_*.txt > Centromere_summary.txt
rm Centromere_summary_Shannon.txt
cat ~/scratch/repeats/output_chromosomes/${SPP_Hap}/Chr*/*Centromere_MIN_Shannon.txt > Centromere_summary_Shannon.txt
grep "cent25 " Centromere_summary_Shannon.txt > Centromere_summary_Shannon_25.txt
grep "cent100 " Centromere_summary_Shannon.txt > Centromere_summary_Shannon_100.txt
grep "cent250 " Centromere_summary_Shannon.txt > Centromere_summary_Shannon_250.txt
grep "cent500 " Centromere_summary_Shannon.txt > Centromere_summary_Shannon_500.txt
grep "cent1000 " Centromere_summary_Shannon.txt > Centromere_summary_Shannon_1000.txt
grep "centwind " Centromere_summary_Shannon_35_no_telo.txt > Centromere_summary_Shannon_wind_35_no_telo.txt
Use the run_summary_plots_range function:
# DNA walks for HA412 and HA89
# HA 412 Chr 5 37 130 000 - 37 150 000
# HA 89 Chr 5 35 520 000 - 35 550 000
# HA 89 Chr 5 38 255 000 - 38 275 000
inpath="~/scratch/repeats/input_chromosomes/Ha412/chromosome_files/"
fname= "Ha412_H0"
chromosome="Chr05"
# spectra
startbp=35005001
endbp=39000000
#----------------------
inpath="~/scratch/repeats/input_chromosomes/Sunflower/chromosome_files/"
fname= "Sunflower_H0"
chromosome="ChrHa8905"
# spectra
startbp=35005001
endbp=39000000
#----------------------
library(RepeatOBserverV1)
outpath="~/scratch/repeats/output_chromosomes"
x_cpu=1
pflag=FALSE
writeflag=FALSE
plotflag=FALSE
nam_list0 <- list.files(inpath)
nam_list1 <- tools::file_path_sans_ext(nam_list0)
nam_list1
nam_list2 <- stringr::str_split(nam_list1, "_", simplify =TRUE)
nam_list3 <- stringr::str_split(nam_list2[,3], "part", simplify =TRUE)
chr_list <- nam_list3[,1]
chr_list
# to plot the DNAwalks and Fourier transform heatmaps in the startbp to endbp range above
run_summary_plots_range(fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath, startbp=startbp, endbp=endbp)
Use the largeimagesub_NEW function:
# Example Fruit Fly
inpath="~/scratch/repeats/input_chromosomes/Fly/chromosome_files/"
fname= "Fly_H0"
chromosome="ChrDlow77"
part="part01"
nam="Fly_H0_ChrDlow77part01"
#----------------------
library(RepeatOBserverV1)
outpath="~/scratch/repeats/output_chromosomes"
x_cpu=1
pflag=FALSE
writeflag=FALSE
plotflag=FALSE
nam_list0 <- list.files(inpath)
nam_list1 <- tools::file_path_sans_ext(nam_list0)
nam_list1
nam_list2 <- stringr::str_split(nam_list1, "_", simplify =TRUE)
nam_list3 <- stringr::str_split(nam_list2[,3], "part", simplify =TRUE)
chr_list <- nam_list3[,1]
chr_list
# writes out file for DNA walk and All_spec (the Fourier transform)
write_All_spec_DNAwalk(nam=nam, fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
# read in the All_spec part of interest
All_spec0<-base::as.matrix(utils::read.table(paste0(outpath,"/", fname,"/",chromosome,"/",fname, "_", chromosome,part,"_All_spec.txt"), check.names = FALSE))
# remove blank/zero columns in All_spec
colnames(All_spec0)[ncol(All_spec0)]
# https://stackoverflow.com/questions/21530168/remove-columns-with-zero-values-from-a-dataframe
All_spec0[,which(colSums(All_spec0, na.rm = FALSE, dims = 1) < 1e-5)] <- NA
All_spec <- All_spec0[,which(!is.na(colSums(All_spec0 != 0)) & colSums(All_spec0 != 0) > 0)]
# https://r-graph-gallery.com/heatmap
#stats::heatmap(All_spec[,c(1:100)], Rowv=FALSE, Colv=FALSE)
pngflag=TRUE
ofi <- paste0(outpath,"/", fname,"/", chromosome,"/", fname, "_", chromosome,part,"_All_spec_")
# set repeat lengths of interest : 17-30 bp
r1=base::c(17,30)
base::cat("\nofi:",ofi,"\n")
chromosome <- paste0(chromosome, part)
if(!base::is.null(r1))largeimagesub_NEW(All_spec,fname=fname, chromosome=chromosome,ofi,rangebp=r1,pngflag = pngflag, repround=1, part=1)
Use the run_long_repeats_NEW function:
library(RepeatOBserverV1)
inpath="~/scratch/repeats/input_chromosomes/Einkorn/chromosome_files/"
fname= "Einkorn_H0"
outpath="~/scratch/repeats/output_chromosomes"
x_cpu=1
pflag=FALSE
writeflag=FALSE
plotflag=FALSE
nam_list0 <- list.files(inpath)
nam_list1 <- tools::file_path_sans_ext(nam_list0)
nam_list1
nam_list2 <- stringr::str_split(nam_list1, "_", simplify =TRUE)
nam_list3 <- stringr::str_split(nam_list2[,3], "part", simplify =TRUE)
chr_list <- nam_list3[,1]
chr_list
# run chromosomes on different cpu
uni_chr_list <- unique(chr_list)
uni_chr_list
for (nam in chr_list){
print(nam)
run_long_repeats_NEW(nam=nam, fname=fname, inpath=inpath, outpath=outpath)
}
# or for a specific chromosome
chromosome="Chraeg1A"
run_long_repeats_NEW(nam=nam, fname=fname, inpath=inpath,outpath=outpath)
If the script above does not run properly on your machine, you can restart RepeatOBserverV1 at any point using the code described here. If an output folder has already been made for a given chromosome, this folder will need to be deleted before the program can be rerun on this chromosome again.
To setup your chromosomes with the proper formatting in the command line for the instructions below.
Change to the directory you want to run the program in:
cd ~/scratch/repeats/
# in this directory you should ahev a input and output directory
mkdir input_chromosomes
mkdir output_chromosomes
cd input_chromosomes; mkdir <Your-spp-name>
cd <Your-spp-name>
Download or copy over your fasta file here and unzip it.
# download or copy over your fasta file here
wget https://ftp.ncbi.nlm.nih.gov/genomes/all/GCA/...
# Unzip the file if needed
gunzip genomic.fna.gz
Fold the fasta file so that it reads in R easily and split it into chromosome fasta files
# fold to 60bp per line to make easier to read into R
module load seqkit/2.3.1
seqkit seq -w 60 genomic.fna > genomic_2.fasta
# make a new directory for the chromosome files
mkdir chromosome_files/
cd ./chromosome_files/
# split the genome into a specifc file for each chromosome
awk '/^>/ { file=substr($1,2) ".fasta" } { print > file }' ../genomic_2.fasta
Remove the small files <5Mbp (e.g. contigs). You can run the program on smaller sequences if desired.
find -type f -size -5000000c -delete
Chromosomes need to be named specifically for this program to work. Rename the chromosomes to speciesname_haplotype_chromosome. Note that these names cannot contain '_' or spaces and must end in '.fasta'.
# check names with list
ls
# adjust the input to rename accordingly to your filenames
rename OW20402 SPP_H0_Chr *
rename .1.fasta .fasta *
Split all the chromosomes into <100Mbp parts. Chromosomes less than 100Mbp will have only one part. This is important for helping the program run on very large chromosomes in R.
# loop to split long chromosomes into parts that are each 100Mbp long
ls > ../list_chromosomes.txt
sed 's/.fasta//g' ../list_chromosomes.txt > ../list_chrnum.txt
while IFS= read -r Chr;
do
split -l 1666666 ${Chr}.fasta ${Chr}part --numeric-suffixes=1
rm ${Chr}.fasta
done < ../list_chrnum.txt
# check character count
wc -c *
# go one directory up and add .fasta to all the new split files
cd ..
find ./chromosome_files/ -type f -exec mv {} {}".fasta" \;
To run the R program on a specific chromosome
In R with the RepeatOBserverV1 package installed run the following after adjusting the fname, chromosome, nam(s), inpath and outpath to match your files.
library(RepeatOBserverV1)
inpath="~/scratch/repeats/input_chromosomes/Arab_COLCEN/chromosome_files/"
fname= "COLCEN_H0"
outpath="~/scratch/repeats/output_chromosomes"
x_cpu=1
pflag=FALSE
writeflag=FALSE
plotflag=FALSE
nam_list0 <- list.files(inpath)
nam_list1 <- tools::file_path_sans_ext(nam_list0)
nam_list1
nam_list2 <- stringr::str_split(nam_list1, "_", simplify =TRUE)
nam_list3 <- stringr::str_split(nam_list2[,3], "part", simplify =TRUE)
chr_list <- nam_list3[,1]
chr_list
# run chromosomes on different cpu
uni_chr_list <- unique(chr_list)
uni_chr_list
nam="Chr4part01"
run_plot_chromosome_parallel(nam=nam, fname=fname, inpath=inpath, outpath=outpath, pflag=pflag, plotflag=plotflag, writeflag=writeflag, x_cpu=x_cpu)
write_All_spec_DNAwalk(nam=nam, fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
nam="Chr4part02"
run_plot_chromosome_parallel(nam=nam, fname=fname, inpath=inpath, outpath=outpath, pflag=pflag, plotflag=plotflag, writeflag=writeflag, x_cpu=x_cpu)
write_All_spec_DNAwalk(nam=nam, fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
# once all parts have run you can merge them
chromosome="Chr4"
merge_spectra(fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
join_chromosome_parts(fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
# make the main plots of the chromosome
run_summary_hist(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
run_diversity_plots(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
run_summary_plots(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
To run as a for loop through all chromosomes. Note that chromosomes that have an output directory already but failed will not rerun until the directory is renamed or deleted.
# run the Fourier transform and DNAwalk conversion
for (nam in chr_list){
print(nam)
run_plot_chromosome_parallel(nam=nam, fname=fname, inpath=inpath, outpath=outpath, pflag=pflag, plotflag=plotflag, writeflag=writeflag, x_cpu=x_cpu)
}
# write out the DNAwalks and the Fourier spectra (All_spec)
for (nam in chr_list){
print(nam)
write_All_spec_DNAwalk(nam=nam, fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
}
# join the chromosome parts back together
for (chromosome in uni_chr_list){
merge_spectra(fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
join_chromosome_parts(fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
}
# make histogram plots, diversity plots, Fourier heatmaps and DNAwalks of the chromosomes
for (chromosome in uni_chr_list){
run_summary_hist(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
run_diversity_plots(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
run_summary_plots(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
}
To run with many cpu and on multiple chromosomes:
# in R
library(RepeatOBserverV1)
inpath="~/scratch/repeats/input_chromosomes/Arab_COLCEN/chromosome_files/"
fname= "COLCEN_H0"
outpath="~/scratch/repeats/output_chromosomes"
x_cpu=15
pflag=FALSE
writeflag=FALSE
plotflag=FALSE
nam_list0 <- list.files(inpath)
nam_list1 <- tools::file_path_sans_ext(nam_list0)
nam_list1
nam_list2 <- stringr::str_split(nam_list1, "_", simplify =TRUE)
nam_list3 <- stringr::str_split(nam_list2[,3], "part", simplify =TRUE)
chr_list <- nam_list3[,1]
chr_list
#--------------------
# run intial spectra if directories do not exist
# if directory failed partway delete it and rerun
for (nam in nam_list1){
run_plot_chromosome_parallel(nam=nam, fname=fname, inpath=inpath, outpath=outpath, pflag=pflag, plotflag=plotflag, writeflag=writeflag, x_cpu=x_cpu)
}
#-----------------------
# writing of summary files on different cpu
nam_write_all_spec <- function(x, nam_list1=nam_list1, chr_list=chr_list, fname=fname, inpath=inpath, outpath=outpath, pflag=pflag, plotflag=plotflag, writeflag=writeflag, x_cpu=x_cpu){
library(RepeatOBserverV1)
nam <<- nam_list1[x]
print(nam)
chromosome <- chr_list[x]
print(chromosome)
write_All_spec_DNAwalk(nam=nam, fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
}
x_cpu=2
cl <- parallel::makeCluster(x_cpu)
results1 <- parallel::parSapply(cl, base::seq_along(c(1:length(nam_list1))), nam_write_all_spec, nam_list1=nam_list1, chr_list=chr_list, fname=fname, inpath=inpath, outpath=outpath, pflag=pflag, plotflag=plotflag, writeflag=writeflag, x_cpu=x_cpu)
#-----------------------
# makes summary files by merging parts of chromosomes
# run chromosomes on different cpu
uni_chr_list <- unique(chr_list)
chromosome_summary <- function(x, uni_chr_list=uni_chr_list, fname=fname, inpath=inpath, outpath=outpath){
library(RepeatOBserverV1)
chromosome <<- uni_chr_list[x]
print(chromosome)
merge_spectra(fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
join_chromosome_parts(fname=fname, chromosome=chromosome, inpath=inpath, outpath=outpath)
}
x_cpu=5
cl <- parallel::makeCluster(x_cpu)
results2 <- parallel::parSapply(cl, base::seq_along(uni_chr_list), chromosome_summary,uni_chr_list=uni_chr_list, fname=fname, inpath=inpath, outpath=outpath)
#---------------------------
# makes summary plots
# run chromosomes on different cpu
chromosome_plot <- function(x, uni_chr_list=uni_chr_list, fname=fname, inpath=inpath, outpath=outpath){
library(RepeatOBserverV1)
chromosome <<- uni_chr_list[x]
print(chromosome)
run_summary_hist(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
run_diversity_plots(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
run_summary_plots(chromosome=chromosome, fname=fname, inpath=inpath, outpath=outpath)
}
x_cpu=5
cl <- parallel::makeCluster(x_cpu)
results2 <- parallel::parSapply(cl, base::seq_along(uni_chr_list), chromosome_plot,uni_chr_list=uni_chr_list, fname=fname, inpath=inpath, outpath=outpath)