TOmicsVis: TranscriptOmics Visualization.
Website: https://benben-miao.github.io/TOmicsVis/
1.2.1 Install required packages from Bioconductor:
# Install required packages from Bioconductor
install.packages("BiocManager")
BiocManager::install(c("ComplexHeatmap", "EnhancedVolcano", "clusterProfiler", "enrichplot", "impute", "preprocessCore", "Mfuzz"))1.2.2 Github: https://github.com/benben-miao/TOmicsVis/
Install from Github:
install.packages("devtools")
devtools::install_github("benben-miao/TOmicsVis")
# Resolve network by GitClone
devtools::install_git("https://gitclone.com/github.com/benben-miao/TOmicsVis.git")1.2.3 CRAN: https://cran.r-project.org/package=TOmicsVis
Install from CRAN:
# Install from CRAN
install.packages("TOmicsVis")Videos Courses: https://space.bilibili.com/34105515/channel/series
Article Introduction: 全解TOmicsVis完美应用于转录组可视化R包
Article Courses: TOmicsVis 转录组学R代码分析及可视化视频
OmicsSuite: Omics Suite Github: https://github.com/omicssuite/
Authors:
# 1. Library TOmicsVis package
library(TOmicsVis)
#> 载入需要的程辑包:e1071
#>
#> Registered S3 method overwritten by 'GGally':
#> method from
#> +.gg ggplot2
#> 载入需要的程辑包:Biobase
#> 载入需要的程辑包:BiocGenerics
#>
#> 载入程辑包:'BiocGenerics'
#> The following objects are masked from 'package:stats':
#>
#> IQR, mad, sd, var, xtabs
#> The following objects are masked from 'package:base':
#>
#> anyDuplicated, aperm, append, as.data.frame, basename, cbind,
#> colnames, dirname, do.call, duplicated, eval, evalq, Filter, Find,
#> get, grep, grepl, intersect, is.unsorted, lapply, Map, mapply,
#> match, mget, order, paste, pmax, pmax.int, pmin, pmin.int,
#> Position, rank, rbind, Reduce, rownames, sapply, setdiff, sort,
#> table, tapply, union, unique, unsplit, which.max, which.min
#> Welcome to Bioconductor
#>
#> Vignettes contain introductory material; view with
#> 'browseVignettes()'. To cite Bioconductor, see
#> 'citation("Biobase")', and for packages 'citation("pkgname")'.
#>
#> 载入程辑包:'DynDoc'
#> The following object is masked from 'package:BiocGenerics':
#>
#> path
# 2. Extra package
# install.packages("ggplot2")
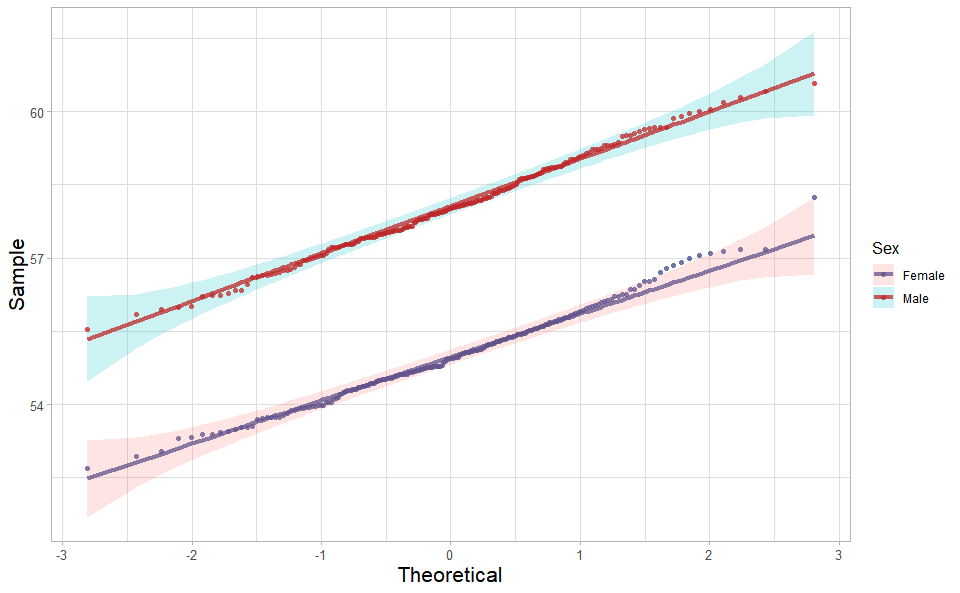
library(ggplot2)Quantile plot for visualizing data distribution.
# 1. Load box_data example datasets
data(quantile_data)
# 2. Run quantile_plot plot function
quantile_plot(
quantile_data,
my_shape = "fill_circle",
point_size = 1.5,
conf_int = TRUE,
conf_level = 0.95,
split_panel = "One_Panel",
legend_pos = "right",
legend_dir = "vertical",
sci_fill_color = "Sci_AAAS",
sci_color_alpha = 0.75,
ggTheme = "theme_light"
)Get help using command ?TOmicsVis::quantile_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/quantile_plot.html.
# Get help with command in R console.
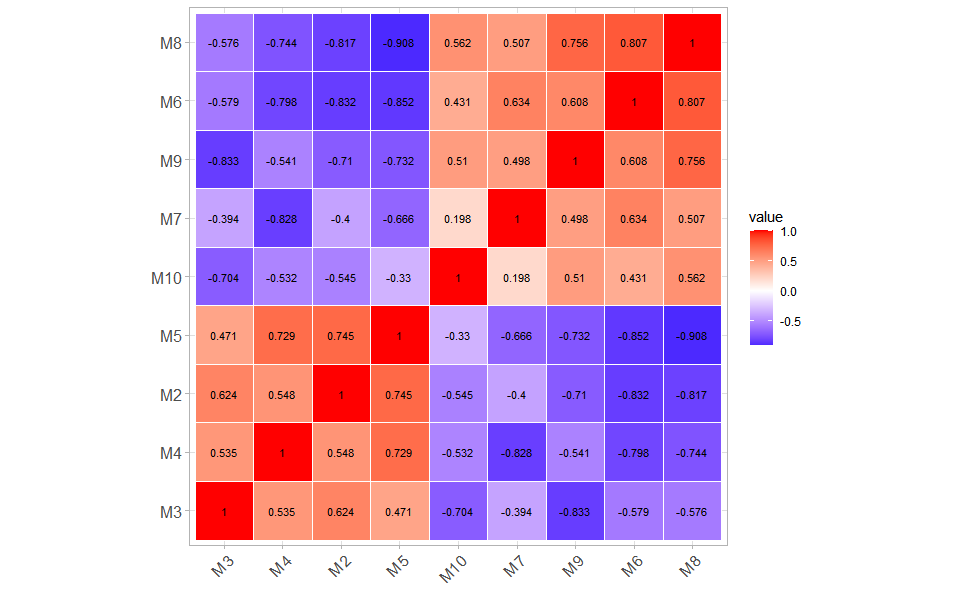
# ?TOmicsVis::quantile_plotCorrelation Heatmap for samples/groups based on Pearson algorithm.
# 1. Load gene_exp example dataset
data(gene_exp)
head(gene_exp)
#> M1 M2 M3 M4 M5 M6 M7 M8
#> RGL4 8.454808 8.019389 8.990836 9.718631 7.908075 4.147051 4.985084 4.576711
#> MPP7 8.690520 8.630346 7.080873 9.838476 8.271824 5.179200 5.200868 3.266993
#> UGCG 8.648366 8.600555 9.431046 7.923021 8.309214 4.902510 5.750804 4.492856
#> CYSTM1 8.628884 9.238677 8.487243 8.958537 7.357109 4.541605 6.370533 4.246651
#> ANXA2 4.983769 6.748022 6.220791 4.719403 3.284346 8.089850 10.637472 7.214912
#> ENDOD1 5.551640 5.406465 4.663785 3.550765 4.103507 8.393991 9.538503 9.069923
#> M9 M10
#> RGL4 4.930349 4.293700
#> MPP7 5.565226 4.300309
#> UGCG 4.659987 3.306275
#> CYSTM1 4.745769 3.449627
#> ANXA2 9.002710 5.123359
#> ENDOD1 8.639664 7.106392
# 2. Run corr_heatmap plot function
corr_heatmap(
gene_exp,
corr_method = "pearson",
cell_shape = "square",
fill_type = "full",
lable_size = 3,
lable_digits = 3,
color_low = "blue",
color_mid = "white",
color_high = "red",
ggTheme = "theme_light"
)
#> Scale for fill is already present.
#> Adding another scale for fill, which will replace the existing scale.Get help using command ?TOmicsVis::corr_heatmap or reference page
https://benben-miao.github.io/TOmicsVis/reference/corr_heatmap.html.
# Get help with command in R console.
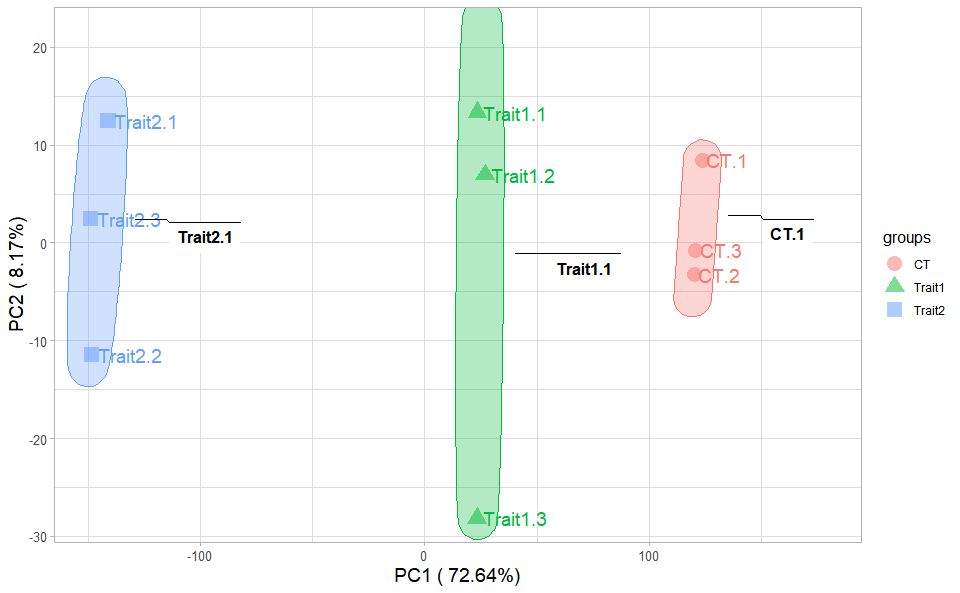
# ?TOmicsVis::corr_heatmapPCA dimensional reduction visualization for RNA-Seq.
# 1. Load pca_sample_gene and pca_group_sample example datasets
data(pca_sample_gene)
data(pca_group_sample)
# 2. Run pca_plot plot function
pca_plot(
pca_sample_gene,
pca_group_sample,
point_size = 5,
text_size = 5,
ellipse_alpha = 0.3,
legend_pos = "right",
legend_dir = "vertical",
ggTheme = "theme_light"
)Get help using command ?TOmicsVis::pca_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/pca_plot.html.
# Get help with command in R console.
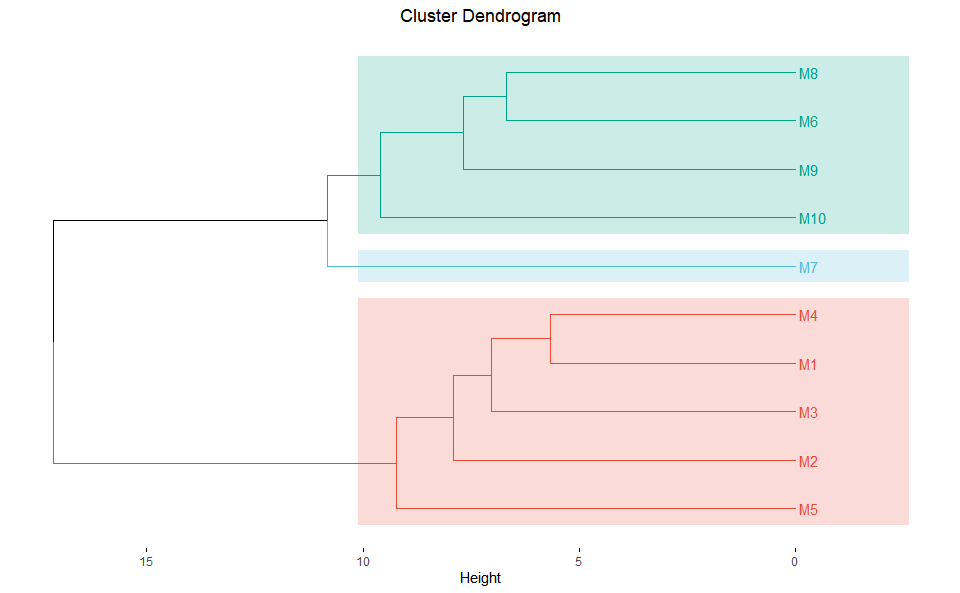
# ?TOmicsVis::pca_plotDendrograms for multiple samples/groups clustering.
# 1. Load example datasets
data(gene_exp)
# 2. Run plot function
dendro_plot(
gene_exp,
dist_method = "euclidean",
hc_method = "average",
tree_type = "rectangle",
k_num = 3,
palette = "npg",
color_labels_by_k = TRUE,
horiz = TRUE,
label_size = 0.8,
line_width = 0.7,
rect = TRUE,
rect_fill = TRUE,
title = "Cluster Dendrogram",
xlab = "",
ylab = "Height"
)
#> Registered S3 method overwritten by 'dendextend':
#> method from
#> rev.hclust veganGet help using command ?TOmicsVis::dendro_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/dendro_plot.html.
# Get help with command in R console.
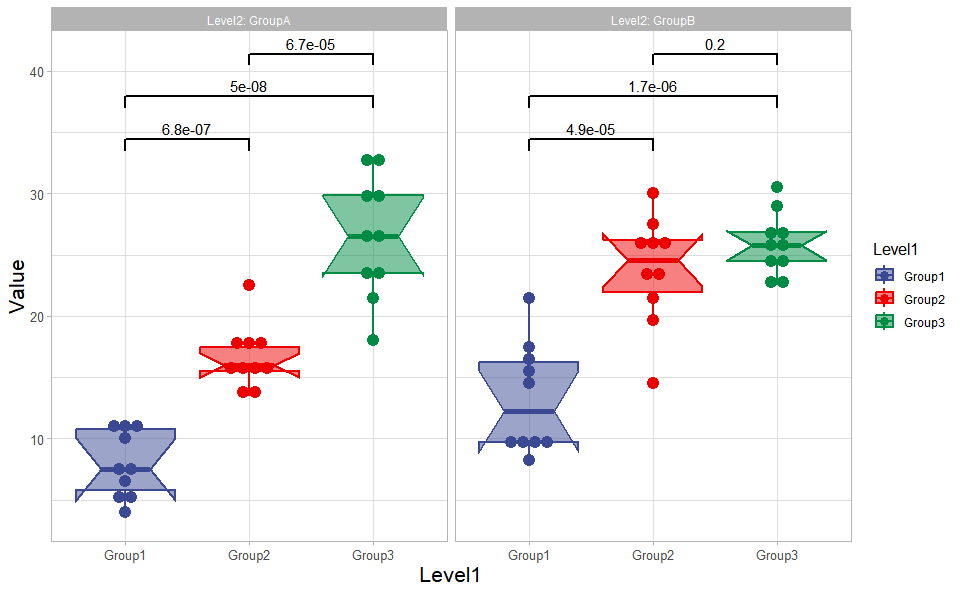
# ?TOmicsVis::dendro_plotBox plot support two levels and multiple groups with P value.
# 1. Load box_data example datasets
data(box_data)
# 2. Run box_plot plot function
box_plot(
box_data,
test_method = "t.test",
test_label = "p.format",
notch = TRUE,
group_level = "Three_Column",
add_element = "dotplot",
my_shape = "fill_circle",
sci_fill_color = "Sci_AAAS",
sci_fill_alpha = 0.5,
sci_color_alpha = 1,
legend_pos = "right",
legend_dir = "vertical",
ggTheme = "theme_light"
)
#> Bin width defaults to 1/30 of the range of the data. Pick better value with
#> `binwidth`.
#> Notch went outside hinges
#> ℹ Do you want `notch = FALSE`?
#> Notch went outside hinges
#> ℹ Do you want `notch = FALSE`?
#> Notch went outside hinges
#> ℹ Do you want `notch = FALSE`?
#> Notch went outside hinges
#> ℹ Do you want `notch = FALSE`?
#> Notch went outside hinges
#> ℹ Do you want `notch = FALSE`?
#> Notch went outside hinges
#> ℹ Do you want `notch = FALSE`?Get help using command ?TOmicsVis::box_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/box_plot.html.
# Get help with command in R console.
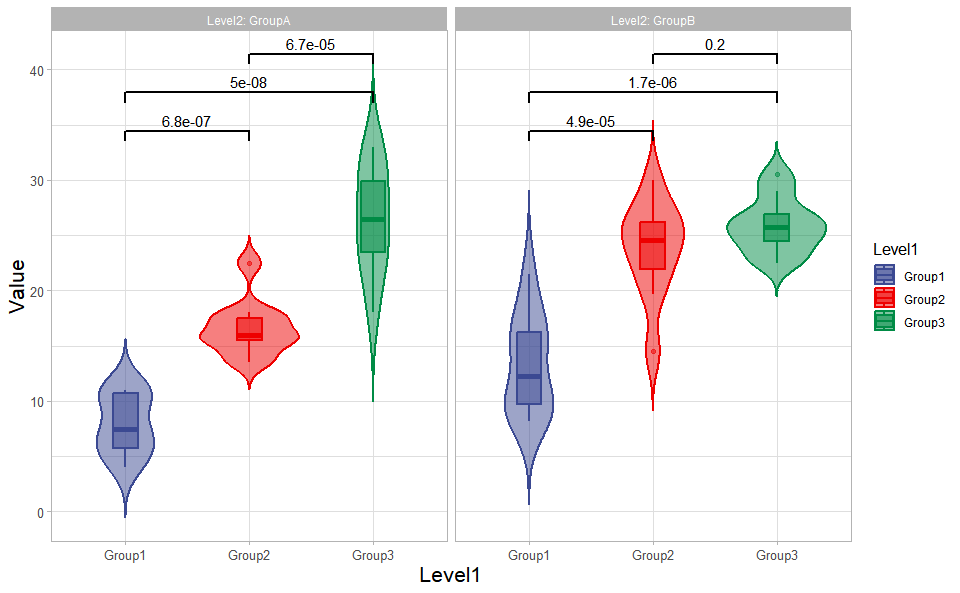
# ?TOmicsVis::box_plotViolin plot support two levels and multiple groups with P value.
# 1. Load box_data example datasets
data(box_data)
# 2. Run violin_plot plot function
violin_plot(
box_data,
test_method = "t.test",
test_label = "p.format",
group_level = "Three_Column",
violin_orientation = "vertical",
add_element = "boxplot",
element_alpha = 0.5,
my_shape = "plus_times",
sci_fill_color = "Sci_AAAS",
sci_fill_alpha = 0.5,
sci_color_alpha = 1,
legend_pos = "right",
legend_dir = "vertical",
ggTheme = "theme_light"
)Get help using command ?TOmicsVis::violin_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/violin_plot.html.
# Get help with command in R console.
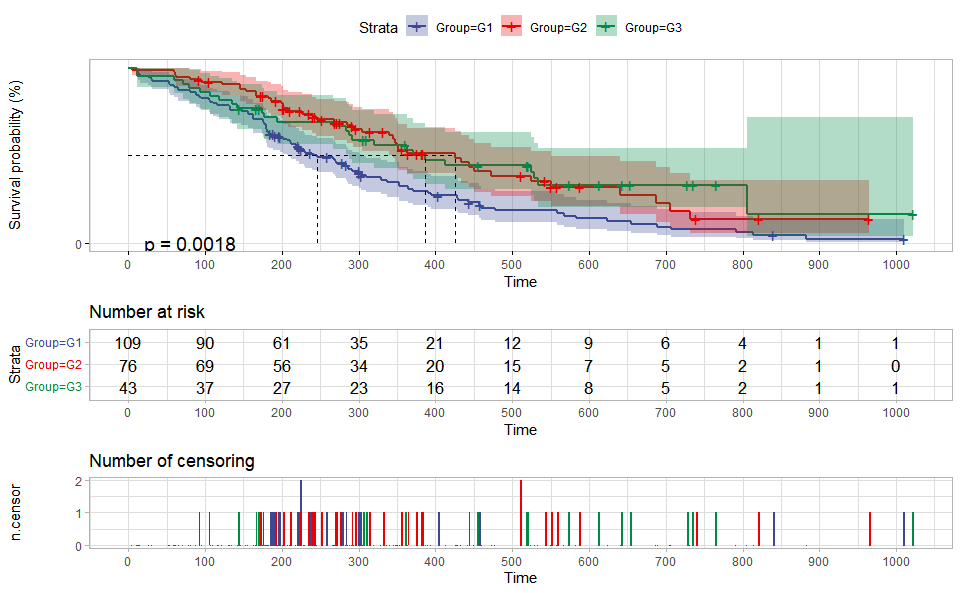
# ?TOmicsVis::violin_plotSurvival plot for analyzing and visualizing survival data.
# 1. Load survival_plot example datasets
data(survival_data)
# 2. Run survival_plot plot function
survival_plot(
survival_data,
curve_function = "pct",
conf_inter = TRUE,
interval_style = "ribbon",
risk_table = TRUE,
num_censor = TRUE,
sci_palette = "aaas",
ggTheme = "theme_light",
x_start = 0,
y_start = 0,
y_end = 100,
x_break = 100,
y_break = 25
)Get help using command ?TOmicsVis::survival_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/survival_plot.html.
# Get help with command in R console.
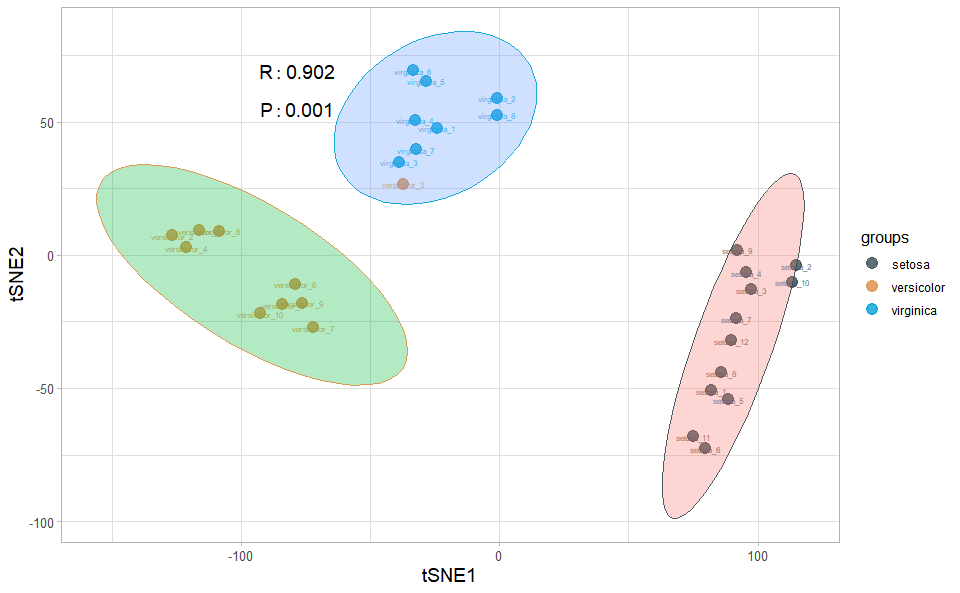
# ?TOmicsVis::survival_plotTSNE plot for analyzing and visualizing TSNE algorithm.
# 1. Load tsne_plot example datasets
data(tsne_data)
# 2. Run tsne_plot plot function
tsne_plot(
tsne_data,
seed = 5,
point_size = 4,
point_alpha = 0.8,
text_size = 2,
text_alpha = 0.8,
ci_level = 0.95,
ellipse_alpha = 0.3,
sci_fill_color = "Sci_JAMA",
sci_color_alpha = 0.9,
legend_pos = "right",
legend_dir = "vertical",
ggTheme = "theme_light"
)Get help using command ?TOmicsVis::tsne_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/tsne_plot.html.
# Get help with command in R console.
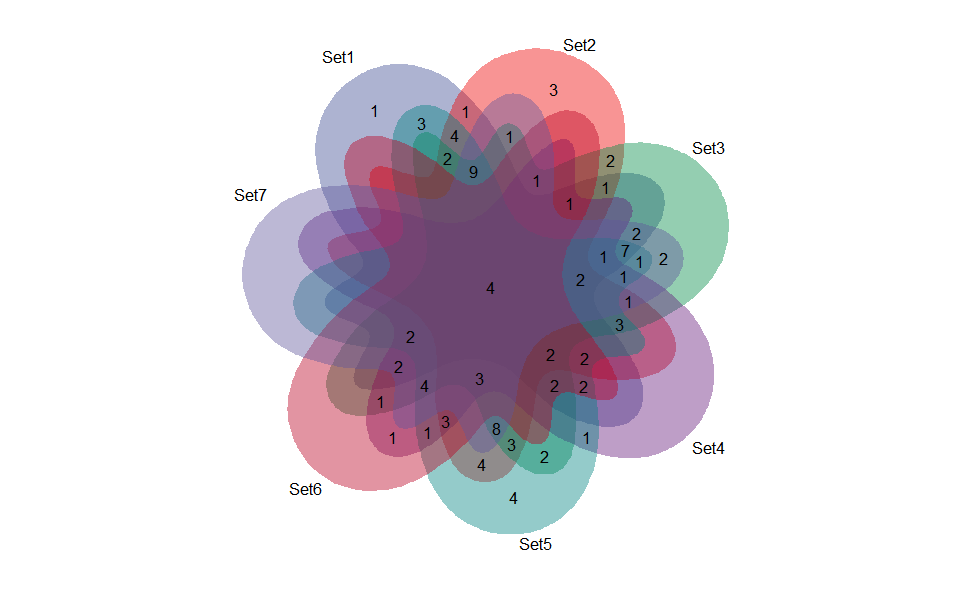
# ?TOmicsVis::tsne_plotVenn plot for stat common and unique gene among multiple sets.
# 1. Load venn_data example datasets
data(venn_data)
# 2. Run venn_plot plot function
venn_plot(
venn_data,
line_type = "blank",
ellipse_shape = "circle",
sci_fill_color = "Sci_AAAS",
sci_fill_alpha = 0.65
)Get help using command ?TOmicsVis::venn_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/venn_plot.html.
# Get help with command in R console.
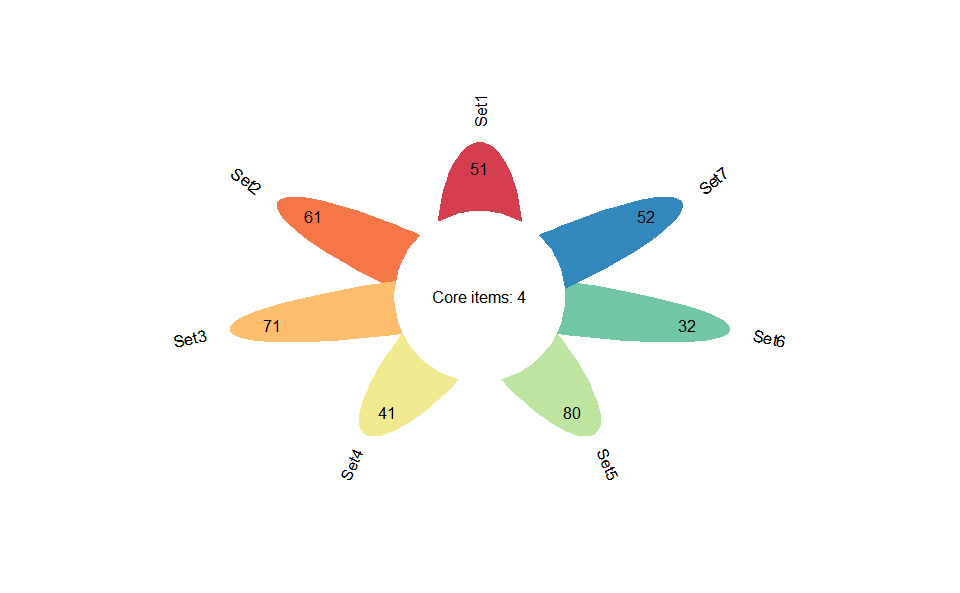
# ?TOmicsVis::venn_plotVenn plot for stat common and unique gene among multiple sets.
# 1. Load example datasets
data(venn_data)
# 2. Run plot function
flower_plot(
venn_data,
angle = 90,
a = 0.5,
b = 2,
r = 1,
ellipse_col_pal = "Spectral",
circle_col = "white",
label_text_cex = 1
)Get help using command ?TOmicsVis::flower_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/flower_plot.html.
# Get help with command in R console.
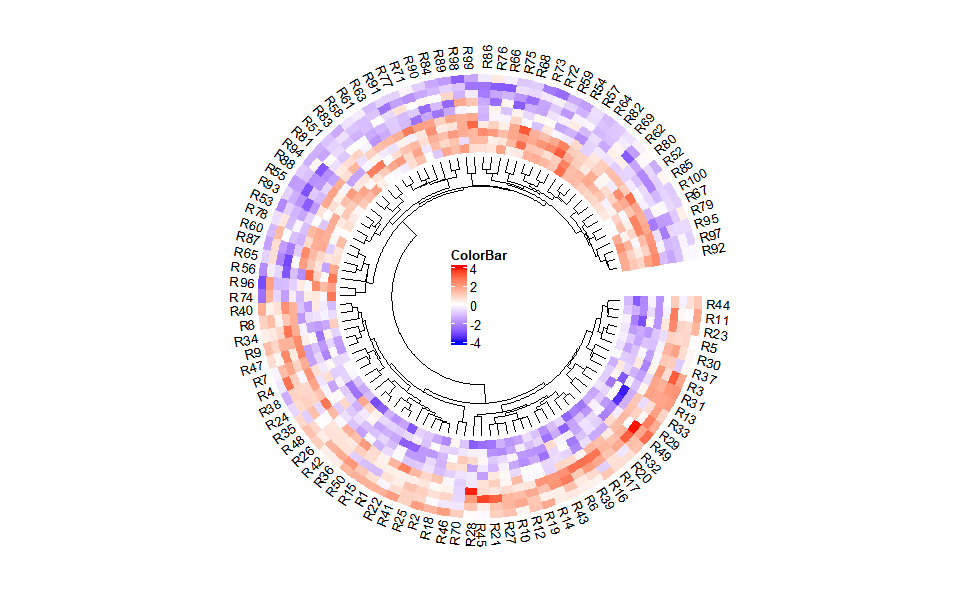
# ?TOmicsVis::flower_plotCircos heatmap plot for visualizing gene expressing in multiple samples.
# 1. Load circos_heatmap_data example datasets
data(circos_heatmap_data)
# 2. Run circos_heatmap plot function
circos_heatmap(
circos_heatmap_data,
low_color = "#0000ff",
mid_color = "#ffffff",
high_color = "#ff0000",
gap_size = 10,
cluster_method = "complete",
distance_method = "euclidean",
dend_height = 0.2,
rowname_size = 0.8
)Get help using command ?TOmicsVis::circos_heatmap or reference page
https://benben-miao.github.io/TOmicsVis/reference/circos_heatmap.html.
# Get help with command in R console.
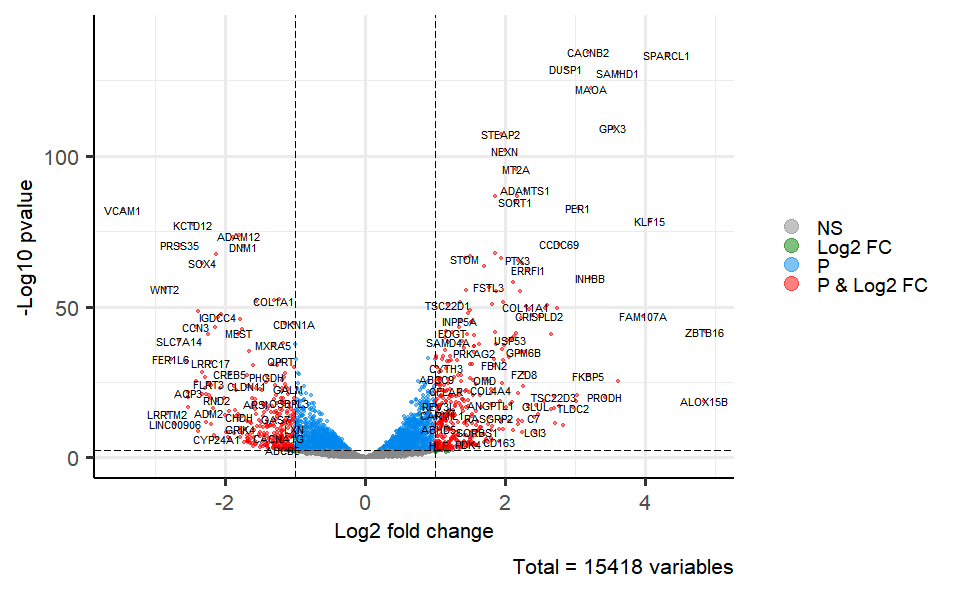
# ?TOmicsVis::circos_heatmapVolcano plot for visualizing differentailly expressed genes.
# 1. Load deg_data example datasets
data(deg_data)
# 2. Run volcano_plot plot function
volcano_plot(
deg_data,
log2fc_cutoff = 1,
pq_value = "pvalue",
pq_cutoff = 0.005,
cutoff_line = "longdash",
point_shape = "large_circle",
point_size = 1,
point_alpha = 0.5,
color_normal = "#888888",
color_log2fc = "#008000",
color_pvalue = "#0088ee",
color_Log2fc_p = "#ff0000",
label_size = 3,
boxed_labels = FALSE,
draw_connectors = FALSE,
legend_pos = "right"
)Get help using command ?TOmicsVis::volcano_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/volcano_plot.html.
# Get help with command in R console.
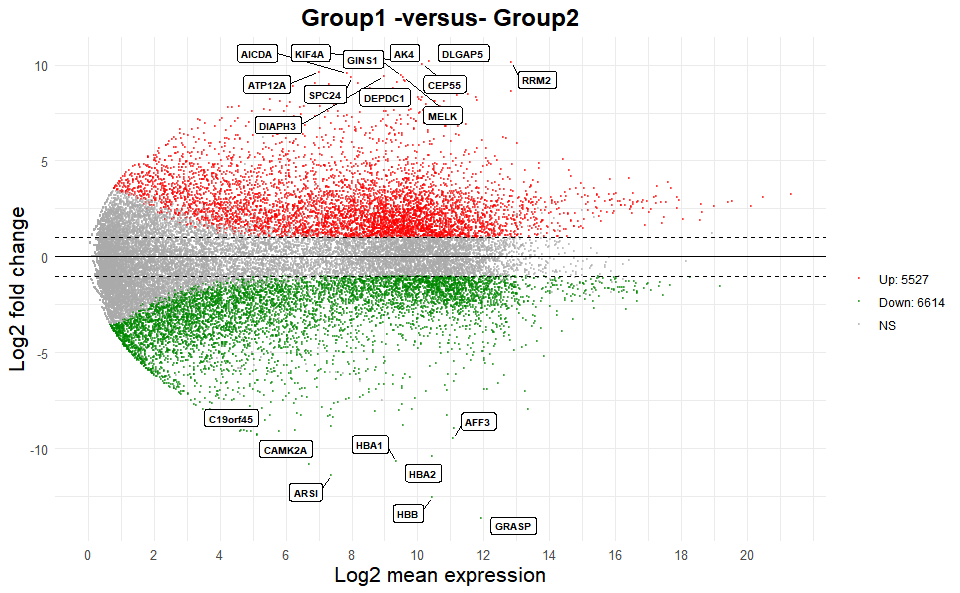
# ?TOmicsVis::volcano_plotMversusA plot for visualizing differentially expressed genes.
# 1. Load deg_data example datasets
data(deg_data2)
# 2. Run volcano_plot plot function
ma_plot(
deg_data2,
foldchange = 2,
fdr_value = 0.05,
point_size = 0.5,
color_up = "#FF0000",
color_down = "#008800",
color_alpha = 0.5,
top_method = "fc",
top_num = 20,
label_size = 8,
label_box = TRUE,
title = "Group1 -versus- Group2",
xlab = "Log2 mean expression",
ylab = "Log2 fold change",
ggTheme = "theme_minimal"
)Get help using command ?TOmicsVis::ma_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/ma_plot.html.
# Get help with command in R console.
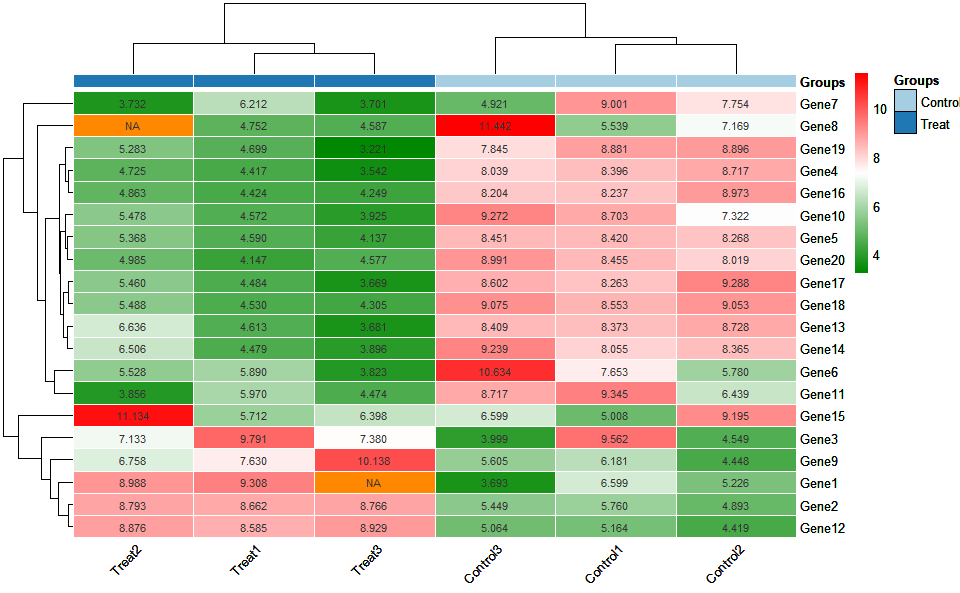
# ?TOmicsVis::ma_plotHeatmap group for visualizing grouped gene expression data.
# 1. Load example datasets
data(heatmap_group_data)
head(heatmap_group_data)
#> V2 V3 V4 V5 V6 V7
#> Groups Control Control Control Treat Treat Treat
#> GeneID Control1 Control2 Control3 Treat1 Treat2 Treat3
#> Gene1 6.59934411 5.226266025 3.693287538 9.308119032 8.987864851 <NA>
#> Gene2 5.760380377 4.892783021 5.448923917 8.66208104 8.793319848 8.765914637
#> Gene3 9.561905115 4.549168157 3.998654922 9.790770004 7.133187551 7.37959102
#> Gene4 8.396409316 8.71705522 8.03906411 4.417013007 4.725269731 3.542216879
# 2. Run heatmap_group plot function
heatmap_group(
data = heatmap_group_data,
scale_data = "none",
clust_method = "complete",
border_show = TRUE,
value_show = TRUE,
low_color = "#00880088",
mid_color = "#ffffff",
high_color = "#ff000088",
na_color = "#ff8800",
x_angle = 45
)Get help using command ?TOmicsVis::heatmap_group or reference page
https://benben-miao.github.io/TOmicsVis/reference/heatmap_group.html.
# Get help with command in R console.
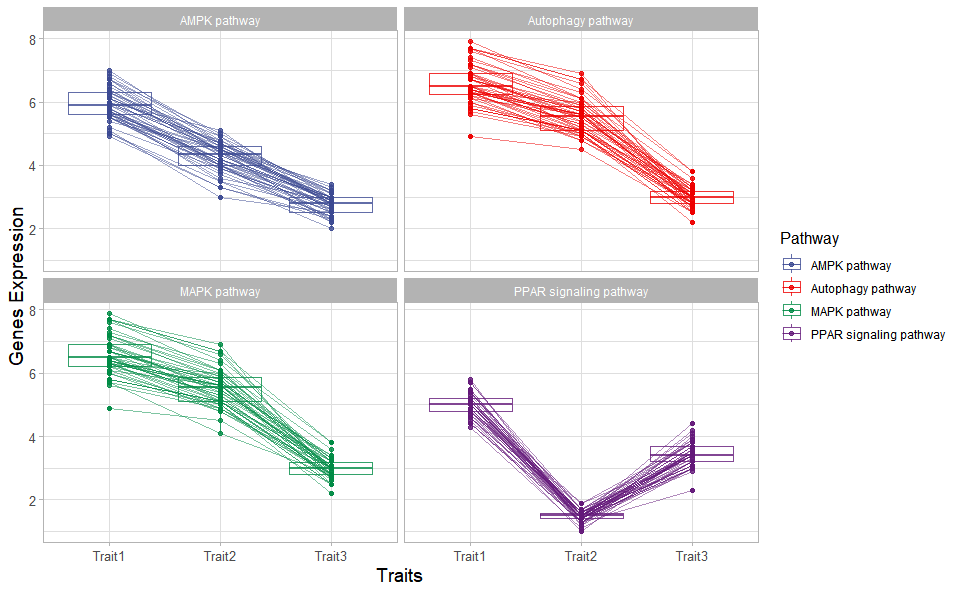
# ?TOmicsVis::heatmap_groupTrend plot for visualizing gene expression trend profile in multiple traits.
# 1. Load chord_data example datasets
data(trend_data)
# 2. Run trend_plot plot function
trend_plot(
trend_data,
scale_method = "globalminmax",
miss_value = "exclude",
line_alpha = 0.5,
show_points = TRUE,
show_boxplot = TRUE,
num_column = 2,
xlab = "Traits",
ylab = "Genes Expression",
sci_fill_color = "Sci_AAAS",
sci_fill_alpha = 0.8,
sci_color_alpha = 0.8,
legend_pos = "right",
legend_dir = "vertical",
ggTheme = "theme_light"
)Get help using command ?TOmicsVis::trend_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/trend_plot.html.
# Get help with command in R console.
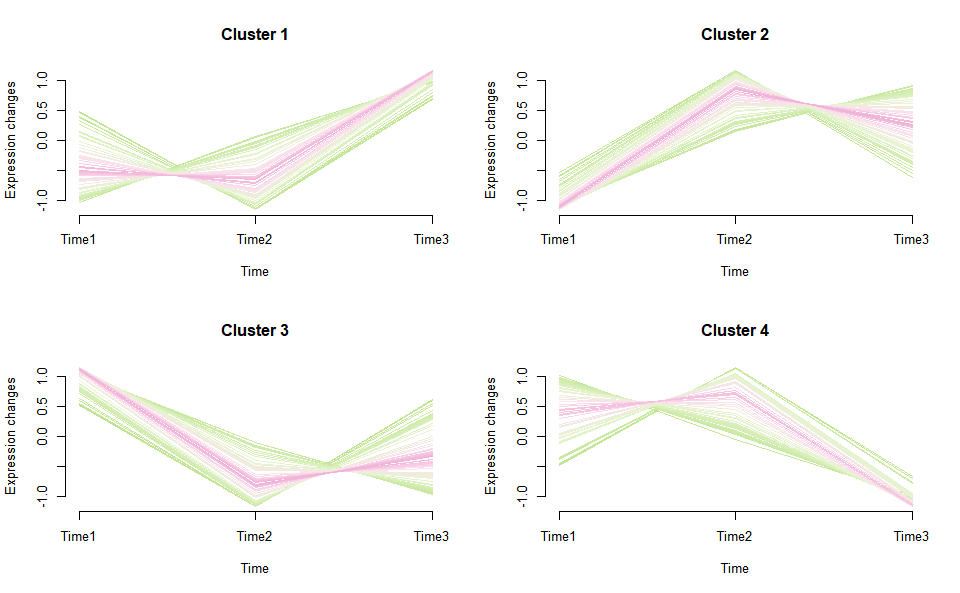
# ?TOmicsVis::trend_plotGene cluster trend plot for visualizing gene expression trend profile in multiple samples.
# 1. Load example datasets
data(gene_cluster_data)
# 2. Run plot function
gene_cluster_trend(
gene_cluster_data,
thres = 0.25,
min_std = 0.2,
palette = "PiYG",
cluster_num = 4
)
#> 0 genes excluded.
#> 12 genes excluded.#> NULL
Get help using command ?TOmicsVis::gene_cluster_trend or reference
page
https://benben-miao.github.io/TOmicsVis/reference/gene_cluster_trend.html.
# Get help with command in R console.
# ?TOmicsVis::gene_cluster_trendGene cluster trend plot for visualizing gene expression trend profile in multiple samples.
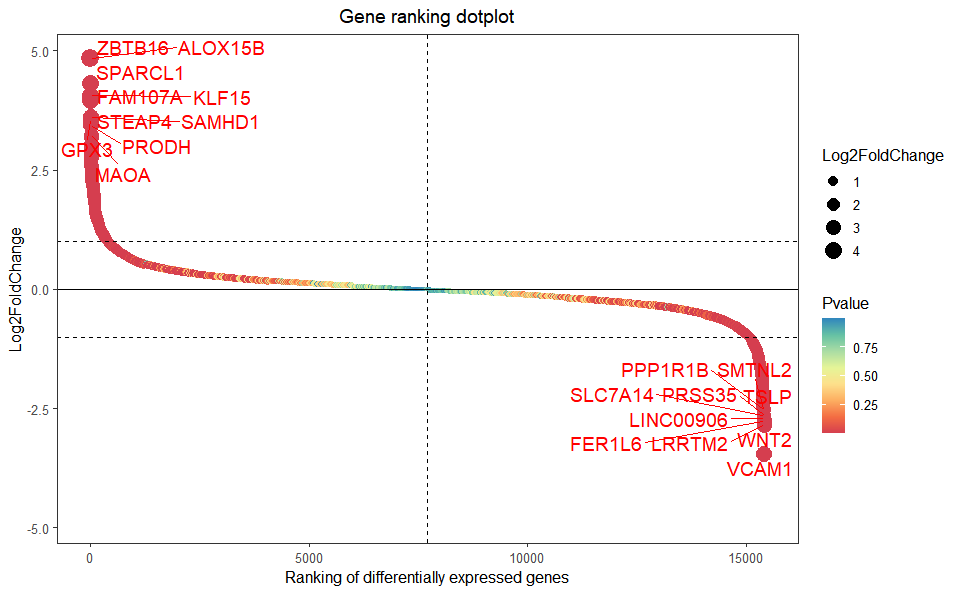
# 1. Load example datasets
data(deg_data)
# 2. Run plot function
gene_rank_plot(
data = deg_data,
log2fc = 1,
palette = "Spectral",
top_n = 10,
genes_to_label = NULL,
label_size = 5,
base_size = 12,
title = "Gene ranking dotplot",
xlab = "Ranking of differentially expressed genes",
ylab = "Log2FoldChange"
)Get help using command ?TOmicsVis::gene_rank_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/gene_rank_plot.html.
# Get help with command in R console.
# ?TOmicsVis::gene_rank_plotWGCNA analysis pipeline for RNA-Seq.
# 1. Load wgcna_pipeline example datasets
data(wgcna_gene_exp)
data(wgcna_sample_group)
# 2. Run wgcna_pipeline plot function
# wgcna_pipeline(wgcna_gene_exp, wgcna_sample_group)Get help using command ?TOmicsVis::wgcna_pipeline or reference page
https://benben-miao.github.io/TOmicsVis/reference/wgcna_pipeline.html.
# Get help with command in R console.
# ?TOmicsVis::wgcna_pipelineNetwork plot for analyzing and visualizing relationship of genes.
# 1. Load example datasets
data(network_data)
head(network_data)
#> node1 node2
#> 1 ABL2 PC-3p-5622_465
#> 2 ABL2 PC-5p-33384_55
#> 3 ABL2 chi-miR-107-3p
#> 4 ABL2 chi-miR-15b-5p
#> 5 CASP2 PC-3p-10204_250
#> 6 CASP2 bta-miR-6123
# 2. Run network_plot plot function
network_plot(
network_data,
calcBy = "degree",
degreeValue = 0.05,
nodeColorNormal = "#00888888",
nodeBorderColor = "#FFFFFF",
nodeColorFrom = "#FF000088",
nodeColorTo = "#00880088",
nodeShapeNormal = "circle",
nodeShapeSpatial = "csquare",
nodeSize = 10,
labelSize = 0.5,
edgeCurved = TRUE,
netLayout = "layout_on_sphere"
)Get help using command ?TOmicsVis::network_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/network_plot.html.
# Get help with command in R console.
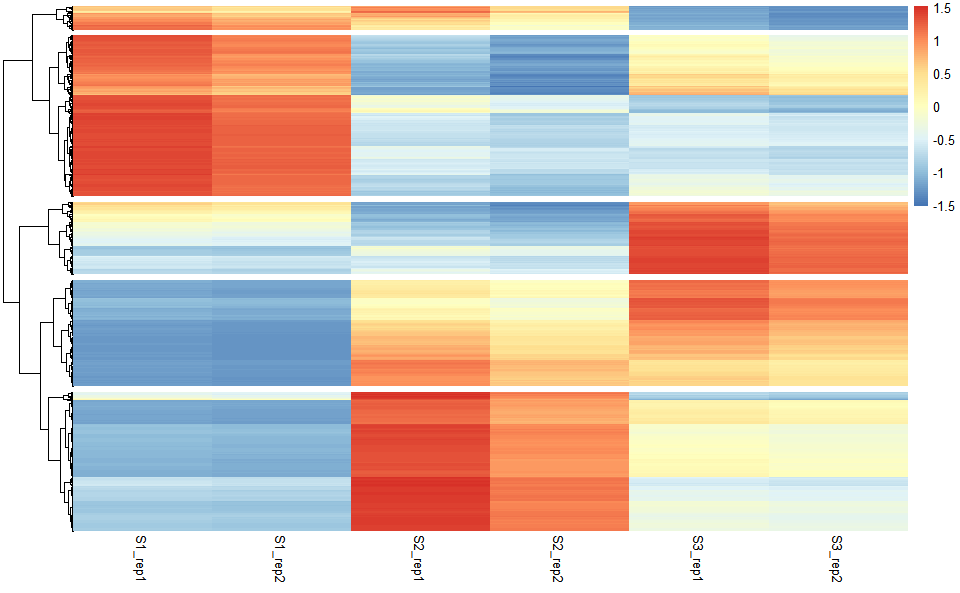
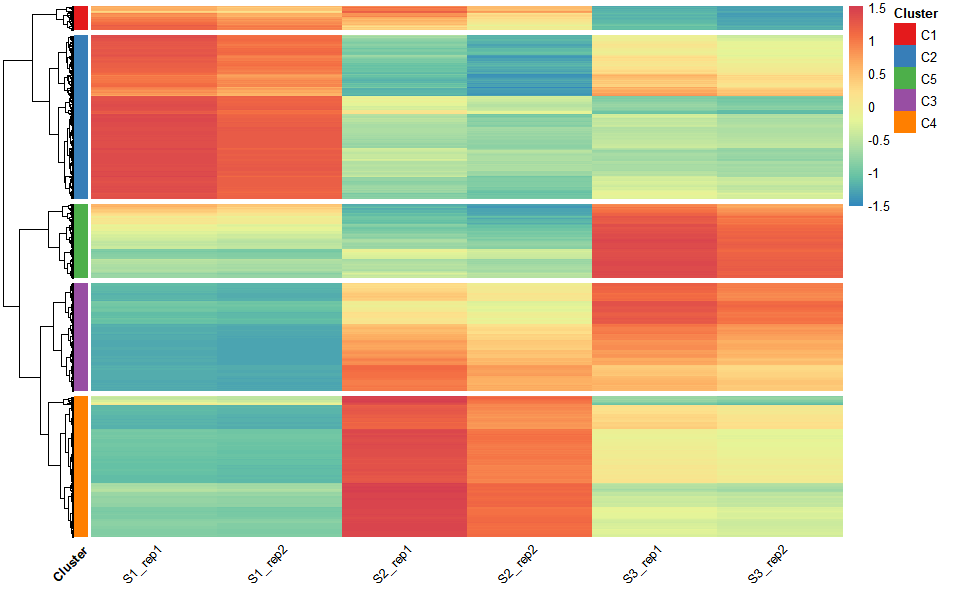
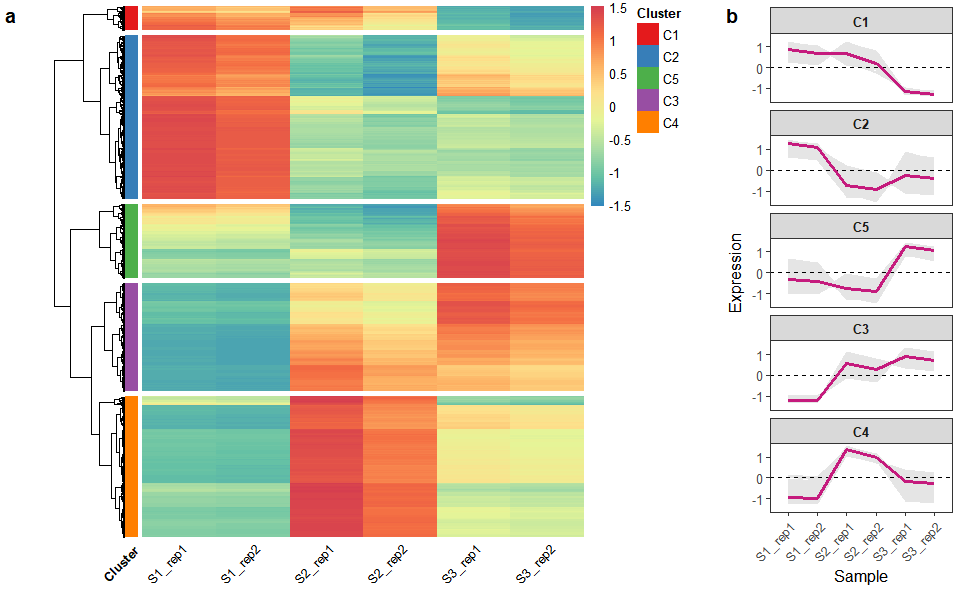
# ?TOmicsVis::network_plotHeatmap cluster plot for visualizing clustered gene expression data.
# 1. Load example datasets
data(gene_exp2)
head(gene_exp2)
#> S1_rep1 S1_rep2 S2_rep1 S2_rep2 S3_rep1 S3_rep2
#> Gene1 316.79234 301.5714 391.75201 344.48179 180.03179 167.99952
#> Gene2 204.21777 194.4057 64.07219 56.34101 93.47004 87.22305
#> Gene3 1049.90451 999.4598 3196.23863 2810.56888 4958.83561 4627.41614
#> Gene4 1413.60565 1345.6862 3356.37956 2951.38662 3947.88238 3684.02909
#> Gene5 98.77808 94.0321 401.04150 352.65038 140.01861 130.66059
#> Gene6 1202.25882 1144.4940 1135.11172 998.14502 784.29842 731.88052
# 2. Run network_plot plot function
heatmap_cluster(
data = gene_exp2,
dist_method = "euclidean",
hc_method = "average",
k_num = 5,
show_rownames = FALSE,
palette = "Spectral",
cluster_pal = "Set1",
gaps_col = NULL,
angle_col = 45,
label_size = 10,
base_size = 12
)#> Using Cluster, gene as id variables
Get help using command ?TOmicsVis::heatmap_cluster or reference page
https://benben-miao.github.io/TOmicsVis/reference/heatmap_cluster.html.
# Get help with command in R console.
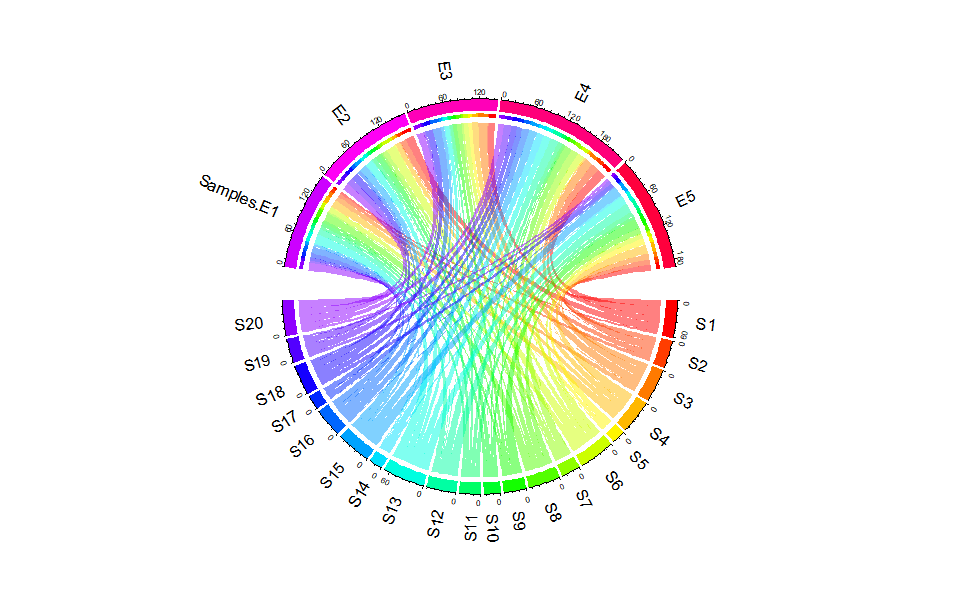
# ?TOmicsVis::heatmap_clusterChord plot for visualizing the relationships of pathways and genes.
# 1. Load chord_data example datasets
data(chord_data)
# 2. Run chord_plot plot function
chord_plot(
chord_data,
multi_colors = "RainbowColors",
color_alpha = 0.5,
link_visible = TRUE,
link_dir = -1,
link_type = "diffHeight",
sector_scale = "Origin",
width_circle = 3,
dist_name = 3,
label_dir = "Vertical",
dist_label = 0.3
)#> rn cn value1 value2 o1 o2 x1 x2 col
#> 1 S1 Samples.E1 4 4 5 20 61 161 #FF00007F
#> 2 S2 Samples.E1 7 7 5 19 45 157 #FF3D007F
#> 3 S3 Samples.E1 9 9 5 18 55 150 #FF7A007F
#> 4 S4 Samples.E1 14 14 5 17 55 141 #FFB8007F
#> 5 S5 Samples.E1 1 1 5 16 22 127 #FFF5007F
#> 6 S6 Samples.E1 10 10 5 15 59 126 #CCFF007F
#> 7 S7 Samples.E1 3 3 5 14 31 116 #8FFF007F
#> 8 S8 Samples.E1 15 15 5 13 52 113 #52FF007F
#> 9 S9 Samples.E1 5 5 5 12 37 98 #14FF007F
#> 10 S10 Samples.E1 7 7 5 11 27 93 #00FF297F
#> 11 S11 Samples.E1 6 6 5 10 35 86 #00FF667F
#> 12 S12 Samples.E1 11 11 5 9 49 80 #00FFA37F
#> 13 S13 Samples.E1 18 18 5 8 69 69 #00FFE07F
#> 14 S14 Samples.E1 1 1 5 7 22 51 #00E0FF7F
#> 15 S15 Samples.E1 5 5 5 6 58 50 #00A3FF7F
#> 16 S16 Samples.E1 12 12 5 5 48 45 #0066FF7F
#> 17 S17 Samples.E1 9 9 5 4 23 33 #0029FF7F
#> 18 S18 Samples.E1 5 5 5 3 50 24 #1400FF7F
#> 19 S19 Samples.E1 4 4 5 2 40 19 #5200FF7F
#> 20 S20 Samples.E1 15 15 5 1 58 15 #8F00FF7F
#> 21 S1 E2 16 16 4 20 57 166 #FF00007F
#> 22 S2 E2 11 11 4 19 38 150 #FF3D007F
#> 23 S3 E2 2 2 4 18 46 139 #FF7A007F
#> 24 S4 E2 9 9 4 17 41 137 #FFB8007F
#> 25 S5 E2 1 1 4 16 21 128 #FFF5007F
#> 26 S6 E2 18 18 4 15 49 127 #CCFF007F
#> 27 S7 E2 8 8 4 14 28 109 #8FFF007F
#> 28 S8 E2 4 4 4 13 37 101 #52FF007F
#> 29 S9 E2 4 4 4 12 32 97 #14FF007F
#> 30 S10 E2 9 9 4 11 20 93 #00FF297F
#> 31 S11 E2 7 7 4 10 29 84 #00FF667F
#> 32 S12 E2 2 2 4 9 38 77 #00FFA37F
#> 33 S13 E2 13 13 4 8 51 75 #00FFE07F
#> 34 S14 E2 2 2 4 7 21 62 #00E0FF7F
#> 35 S15 E2 13 13 4 6 53 60 #00A3FF7F
#> 36 S16 E2 7 7 4 5 36 47 #0066FF7F
#> 37 S17 E2 2 2 4 4 14 40 #0029FF7F
#> 38 S18 E2 13 13 4 3 45 38 #1400FF7F
#> 39 S19 E2 12 12 4 2 36 25 #5200FF7F
#> 40 S20 E2 13 13 4 1 43 13 #8F00FF7F
#> 41 S1 E3 12 12 3 20 41 150 #FF00007F
#> 42 S2 E3 2 2 3 19 27 138 #FF3D007F
#> 43 S3 E3 17 17 3 18 44 136 #FF7A007F
#> 44 S4 E3 12 12 3 17 32 119 #FFB8007F
#> 45 S5 E3 7 7 3 16 20 107 #FFF5007F
#> 46 S6 E3 9 9 3 15 31 100 #CCFF007F
#> 47 S7 E3 4 4 3 14 20 91 #8FFF007F
#> 48 S8 E3 9 9 3 13 33 87 #52FF007F
#> 49 S9 E3 7 7 3 12 28 78 #14FF007F
#> 50 S10 E3 1 1 3 11 11 71 #00FF297F
#> 51 S11 E3 5 5 3 10 22 70 #00FF667F
#> 52 S12 E3 2 2 3 9 36 65 #00FFA37F
#> 53 S13 E3 8 8 3 8 38 63 #00FFE07F
#> 54 S14 E3 2 2 3 7 19 55 #00E0FF7F
#> 55 S15 E3 6 6 3 6 40 53 #00A3FF7F
#> 56 S16 E3 15 15 3 5 29 47 #0066FF7F
#> 57 S17 E3 1 1 3 4 12 32 #0029FF7F
#> 58 S18 E3 13 13 3 3 32 31 #1400FF7F
#> 59 S19 E3 4 4 3 2 24 18 #5200FF7F
#> 60 S20 E3 14 14 3 1 30 14 #8F00FF7F
#> 61 S1 E4 18 18 2 20 29 227 #FF00007F
#> 62 S2 E4 15 15 2 19 25 209 #FF3D007F
#> 63 S3 E4 16 16 2 18 27 194 #FF7A007F
#> 64 S4 E4 3 3 2 17 20 178 #FFB8007F
#> 65 S5 E4 1 1 2 16 13 175 #FFF5007F
#> 66 S6 E4 13 13 2 15 22 174 #CCFF007F
#> 67 S7 E4 15 15 2 14 16 161 #8FFF007F
#> 68 S8 E4 13 13 2 13 24 146 #52FF007F
#> 69 S9 E4 3 3 2 12 21 133 #14FF007F
#> 70 S10 E4 4 4 2 11 10 130 #00FF297F
#> 71 S11 E4 8 8 2 10 17 126 #00FF667F
#> 72 S12 E4 16 16 2 9 34 118 #00FFA37F
#> 73 S13 E4 14 14 2 8 30 102 #00FFE07F
#> 74 S14 E4 14 14 2 7 17 88 #00E0FF7F
#> 75 S15 E4 16 16 2 6 34 74 #00A3FF7F
#> 76 S16 E4 12 12 2 5 14 58 #0066FF7F
#> 77 S17 E4 10 10 2 4 11 46 #0029FF7F
#> 78 S18 E4 11 11 2 3 19 36 #1400FF7F
#> 79 S19 E4 14 14 2 2 20 25 #5200FF7F
#> 80 S20 E4 11 11 2 1 16 11 #8F00FF7F
#> 81 S1 E5 11 11 1 20 11 192 #FF00007F
#> 82 S2 E5 10 10 1 19 10 181 #FF3D007F
#> 83 S3 E5 11 11 1 18 11 171 #FF7A007F
#> 84 S4 E5 17 17 1 17 17 160 #FFB8007F
#> 85 S5 E5 12 12 1 16 12 143 #FFF5007F
#> 86 S6 E5 9 9 1 15 9 131 #CCFF007F
#> 87 S7 E5 1 1 1 14 1 122 #8FFF007F
#> 88 S8 E5 11 11 1 13 11 121 #52FF007F
#> 89 S9 E5 18 18 1 12 18 110 #14FF007F
#> 90 S10 E5 6 6 1 11 6 92 #00FF297F
#> 91 S11 E5 9 9 1 10 9 86 #00FF667F
#> 92 S12 E5 18 18 1 9 18 77 #00FFA37F
#> 93 S13 E5 16 16 1 8 16 59 #00FFE07F
#> 94 S14 E5 3 3 1 7 3 43 #00E0FF7F
#> 95 S15 E5 18 18 1 6 18 40 #00A3FF7F
#> 96 S16 E5 2 2 1 5 2 22 #0066FF7F
#> 97 S17 E5 1 1 1 4 1 20 #0029FF7F
#> 98 S18 E5 8 8 1 3 8 19 #1400FF7F
#> 99 S19 E5 6 6 1 2 6 11 #5200FF7F
#> 100 S20 E5 5 5 1 1 5 5 #8F00FF7F
Get help using command ?TOmicsVis::chord_plot or reference page
https://benben-miao.github.io/TOmicsVis/reference/chord_plot.html.
# Get help with command in R console.
# ?TOmicsVis::chord_plotGO enrichment analysis based on GO annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(go_anno)
head(go_anno)
#> id
#> 1 gene_1
#> 2 gene_2
#> 3 gene_3
#> 4 gene_4
#> 5 gene_5
#> 6 gene_6
#> biological_process
#> 1 GO:0015986(ATP synthesis coupled proton transport)
#> 2 GO:0071236(cellular response to antibiotic);GO:0071493(cellular response to UV-B);GO:0008630(intrinsic apoptotic signaling pathway in response to DNA damage);GO:0010043(response to zinc ion)
#> 3 GO:0009058(biosynthetic process)
#> 4 GO:0006096(glycolytic process)
#> 5 <NA>
#> 6 GO:0006412(translation)
#> cellular_component
#> 1 "GO:0000276(mitochondrial proton-transporting ATP synthase complex, coupling factor F(o))"
#> 2 GO:0005737(cytoplasm)
#> 3 <NA>
#> 4 GO:0000015(phosphopyruvate hydratase complex)
#> 5 <NA>
#> 6 GO:0022625(cytosolic large ribosomal subunit)
#> molecular_function
#> 1 GO:0047624(adenosine-tetraphosphatase activity);GO:0015078(proton transmembrane transporter activity);GO:0016887(ATPase activity)
#> 2 "GO:0046872(metal ion binding);GO:0003680(AT DNA binding);GO:0008301(DNA binding, bending);GO:0042277(peptide binding);GO:0008270(zinc ion binding)"
#> 3 GO:0003824(catalytic activity)
#> 4 GO:0000287(magnesium ion binding);GO:0004634(phosphopyruvate hydratase activity)
#> 5 GO:0005319(lipid transporter activity)
#> 6 GO:0003723(RNA binding);GO:0003735(structural constituent of ribosome)
data(go_deg_fc)
head(go_deg_fc)
#> id log2FC
#> 1 gene_14 -1.20
#> 2 gene_15 1.25
#> 3 gene_16 1.30
#> 4 gene_17 1.35
#> 5 gene_18 -1.50
#> 6 gene_20 -1.55
# 2. Run go_enrich analysis function
res <- go_enrich(
go_anno,
go_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 0.5,
qvalue_cutoff = 0.5
)
head(res)
#> ID ontology
#> 1 GO:0000027 biological process
#> 2 GO:0001732 biological process
#> 3 GO:0001944 biological process
#> 4 GO:0003723 molecular function
#> 5 GO:0003735 molecular function
#> 6 GO:0003743 molecular function
#> Description GeneRatio BgRatio
#> 1 ribosomal large subunit assembly 1/11 1/100
#> 2 formation of cytoplasmic translation initiation complex 1/11 1/100
#> 3 vasculature development 1/11 1/100
#> 4 RNA binding 1/11 11/100
#> 5 structural constituent of ribosome 3/11 26/100
#> 6 translation initiation factor activity 1/11 1/100
#> pvalue p.adjust qvalue geneID Count
#> 1 0.1100000 0.1850000 0.1578947 gene_23 1
#> 2 0.1100000 0.1850000 0.1578947 gene_21 1
#> 3 0.1100000 0.1850000 0.1578947 gene_21 1
#> 4 0.7421470 0.7421470 0.6334113 gene_23 1
#> 5 0.5849832 0.6365993 0.5433280 gene_22/gene_23/gene_24 3
#> 6 0.1100000 0.1850000 0.1578947 gene_21 1Get help using command ?TOmicsVis::go_enrich or reference page
https://benben-miao.github.io/TOmicsVis/reference/go_enrich.html.
# Get help with command in R console.
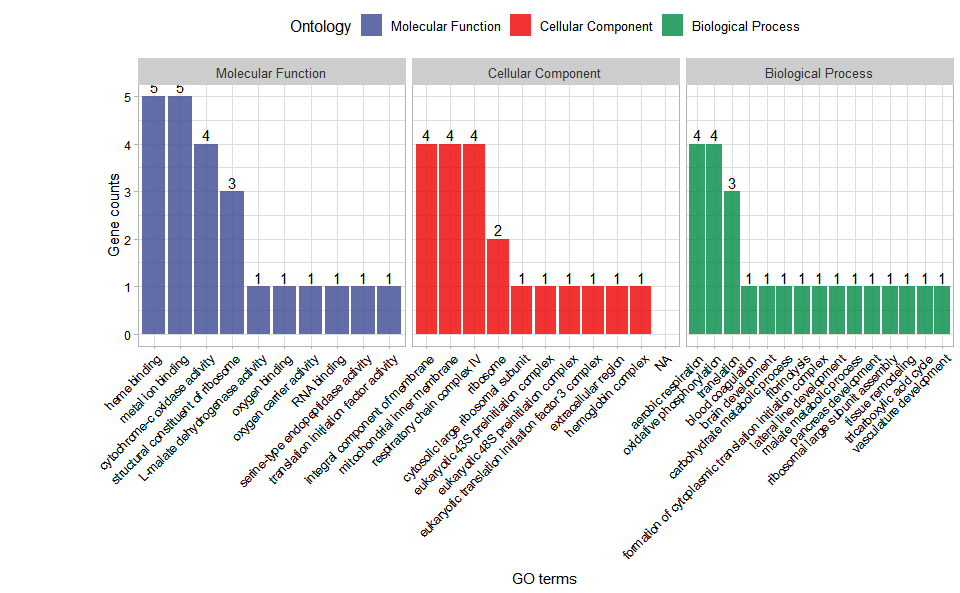
# ?TOmicsVis::go_enrichGO enrichment analysis and stat plot based on GO annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(go_anno)
# head(go_anno)
data(go_deg_fc)
# head(go_deg_fc)
# 2. Run go_enrich_stat analysis function
go_enrich_stat(
go_anno,
go_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 0.5,
qvalue_cutoff = 0.5,
max_go_item = 15,
strip_fill = "#CDCDCD",
xtext_angle = 45,
sci_fill_color = "Sci_AAAS",
sci_fill_alpha = 0.8,
ggTheme = "theme_light"
)Get help using command ?TOmicsVis::go_enrich_stat or reference page
https://benben-miao.github.io/TOmicsVis/reference/go_enrich_stat.html.
# Get help with command in R console.
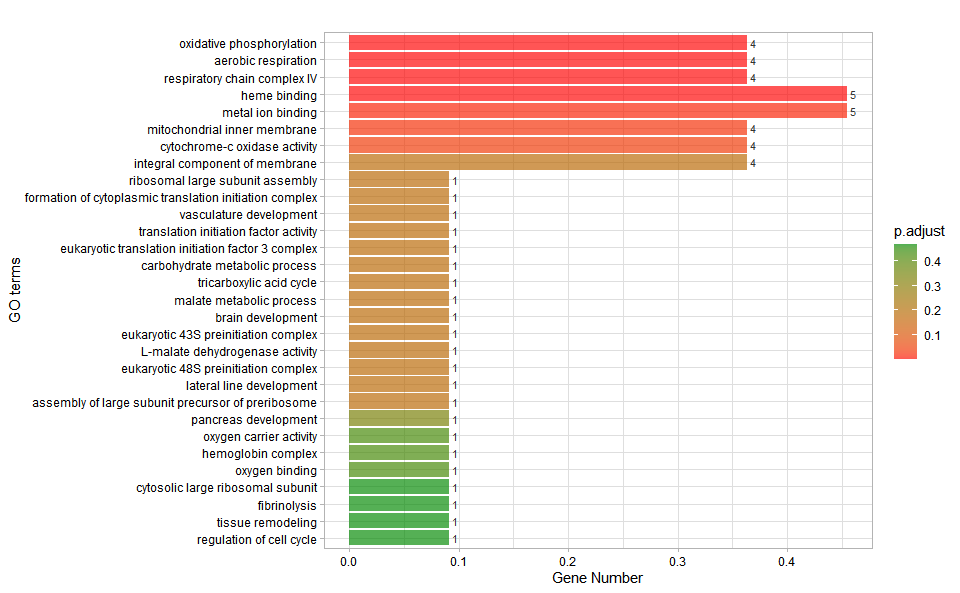
# ?TOmicsVis::go_enrich_statGO enrichment analysis and bar plot based on GO annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(go_anno)
# head(go_anno)
data(go_deg_fc)
# head(go_deg_fc)
# 2. Run go_enrich_bar analysis function
go_enrich_bar(
go_anno,
go_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 0.5,
qvalue_cutoff = 0.5,
sign_by = "p.adjust",
category_num = 30,
font_size = 12,
low_color = "#ff0000aa",
high_color = "#008800aa",
ggTheme = "theme_light"
)
#> Scale for fill is already present.
#> Adding another scale for fill, which will replace the existing scale.Get help using command ?TOmicsVis::go_enrich_bar or reference page
https://benben-miao.github.io/TOmicsVis/reference/go_enrich_bar.html.
# Get help with command in R console.
# ?TOmicsVis::go_enrich_barGO enrichment analysis and dot plot based on GO annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(go_anno)
# head(go_anno)
data(go_deg_fc)
# head(go_deg_fc)
# 2. Run go_enrich_dot analysis function
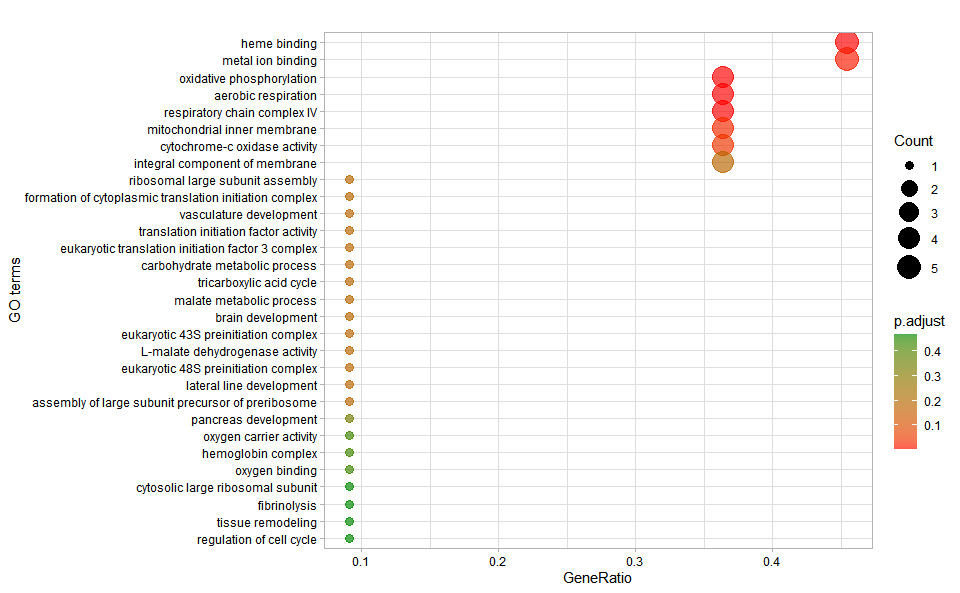
go_enrich_dot(
go_anno,
go_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 0.5,
qvalue_cutoff = 0.5,
sign_by = "p.adjust",
category_num = 30,
font_size = 12,
low_color = "#ff0000aa",
high_color = "#008800aa",
ggTheme = "theme_light"
)
#> Scale for colour is already present.
#> Adding another scale for colour, which will replace the existing scale.Get help using command ?TOmicsVis::go_enrich_dot or reference page
https://benben-miao.github.io/TOmicsVis/reference/go_enrich_dot.html.
# Get help with command in R console.
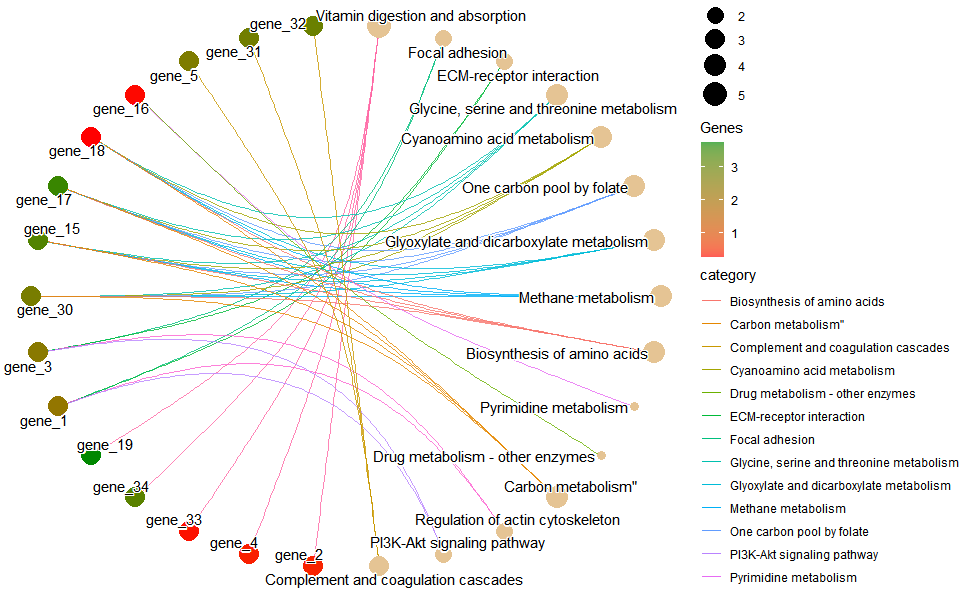
# ?TOmicsVis::go_enrich_dotGO enrichment analysis and tree plot based on GO annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(go_anno)
# head(go_anno)
data(go_deg_fc)
# head(go_deg_fc)
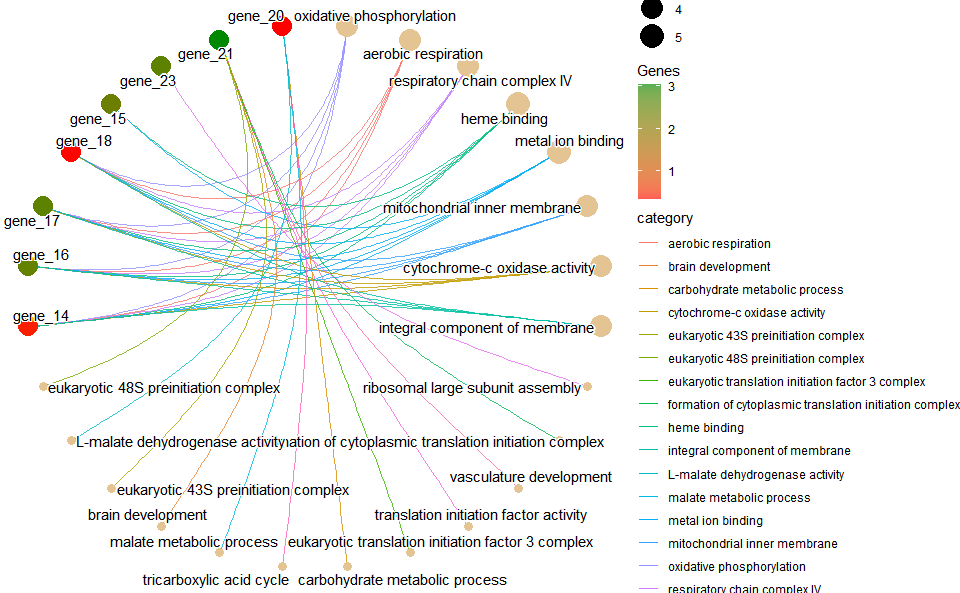
# 2. Run go_enrich_tree analysis function
go_enrich_tree(
go_anno,
go_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 0.5,
qvalue_cutoff = 0.5,
sign_by = "p.adjust",
category_num = 20,
font_size = 4,
low_color = "#ff0000aa",
high_color = "#008800aa",
hclust_method = "complete",
ggTheme = "theme_light"
)Get help using command ?TOmicsVis::go_enrich_tree.
# Get help with command in R console.
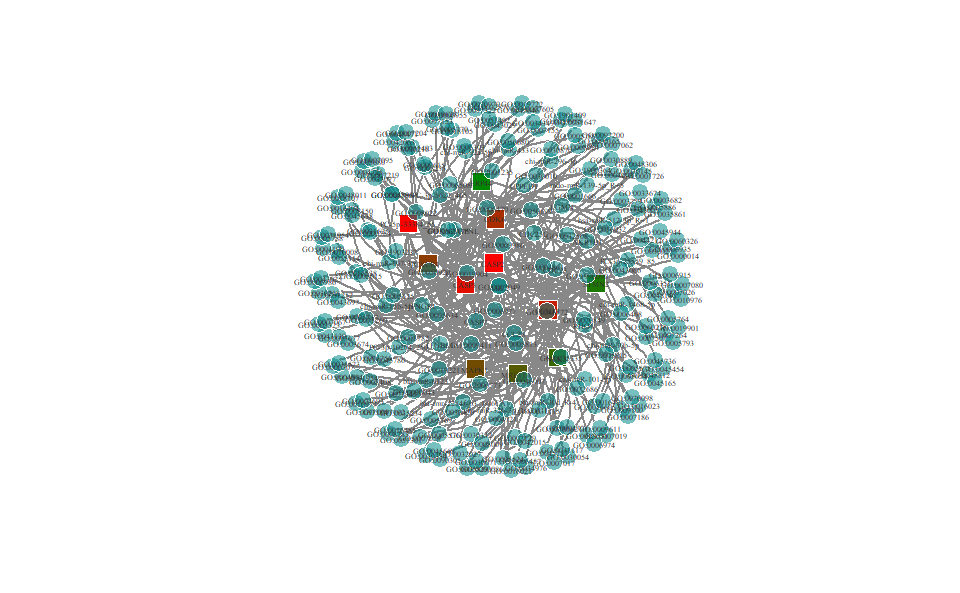
# ?TOmicsVis::go_enrich_treeGO enrichment analysis and net plot based on GO annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(go_anno)
# head(go_anno)
data(go_deg_fc)
# head(go_deg_fc)
# 2. Run go_enrich_net analysis function
go_enrich_net(
go_anno,
go_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 0.5,
qvalue_cutoff = 0.5,
category_num = 20,
net_layout = "circle",
net_circular = TRUE,
low_color = "#ff0000aa",
high_color = "#008800aa"
)
#> Scale for size is already present.
#> Adding another scale for size, which will replace the existing scale.
#> Scale for colour is already present.
#> Adding another scale for colour, which will replace the existing scale.Get help using command ?TOmicsVis::go_enrich_net or reference page
https://benben-miao.github.io/TOmicsVis/reference/go_enrich_net.html.
# Get help with command in R console.
# ?TOmicsVis::go_enrich_netKEGG enrichment analysis based on KEGG annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(kegg_anno)
head(kegg_anno)
#> id
#> 1 gene_1
#> 2 gene_2
#> 3 gene_3
#> 4 gene_4
#> 5 gene_5
#> 6 gene_6
#> kegg_pathway
#> 1 ko04810(Regulation of actin cytoskeleton);ko04510(Focal adhesion);ko04151(PI3K-Akt signaling pathway);ko04512(ECM-receptor interaction)
#> 2 ko04977(Vitamin digestion and absorption)
#> 3 ko04810(Regulation of actin cytoskeleton);ko04510(Focal adhesion);ko04151(PI3K-Akt signaling pathway);ko04512(ECM-receptor interaction)
#> 4 ko04977(Vitamin digestion and absorption)
#> 5 ko04610(Complement and coagulation cascades)
#> 6 ko04142(Lysosome)
data(kegg_deg_fc)
head(kegg_deg_fc)
#> id log2FC
#> 1 gene_1 1.20
#> 2 gene_2 -1.25
#> 3 gene_3 1.30
#> 4 gene_4 -1.35
#> 5 gene_5 1.40
#> 6 gene_30 1.45
# 2. Run go_enrich analysis function
res <- kegg_enrich(
kegg_anno,
kegg_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 1,
qvalue_cutoff = 1
)
head(res)
#> ID Description GeneRatio BgRatio
#> ko04977 ko04977 Vitamin digestion and absorption 5/15 12/93
#> ko04510 ko04510 Focal adhesion 2/15 2/93
#> ko04512 ko04512 ECM-receptor interaction 2/15 2/93
#> "ko00260 "ko00260 Glycine, serine and threonine metabolism 4/15 11/93
#> ko00460 ko00460 Cyanoamino acid metabolism 4/15 11/93
#> ko00670 ko00670 One carbon pool by folate 4/15 11/93
#> pvalue p.adjust qvalue geneID
#> ko04977 0.02247971 0.1227209 0.1033439 gene_2/gene_4/gene_33/gene_34/gene_19
#> ko04510 0.02454418 0.1227209 0.1033439 gene_1/gene_3
#> ko04512 0.02454418 0.1227209 0.1033439 gene_1/gene_3
#> "ko00260 0.07369837 0.1842459 0.1551545 gene_30/gene_15/gene_17/gene_18
#> ko00460 0.07369837 0.1842459 0.1551545 gene_30/gene_15/gene_17/gene_18
#> ko00670 0.07369837 0.1842459 0.1551545 gene_30/gene_15/gene_17/gene_18
#> Count
#> ko04977 5
#> ko04510 2
#> ko04512 2
#> "ko00260 4
#> ko00460 4
#> ko00670 4Get help using command ?TOmicsVis::kegg_enrich or reference page
https://benben-miao.github.io/TOmicsVis/reference/kegg_enrich.html.
# Get help with command in R console.
# ?TOmicsVis::kegg_enrichKEGG enrichment analysis and bar plot based on KEGG annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(kegg_anno)
# head(kegg_anno)
data(kegg_deg_fc)
# head(kegg_deg_fc)
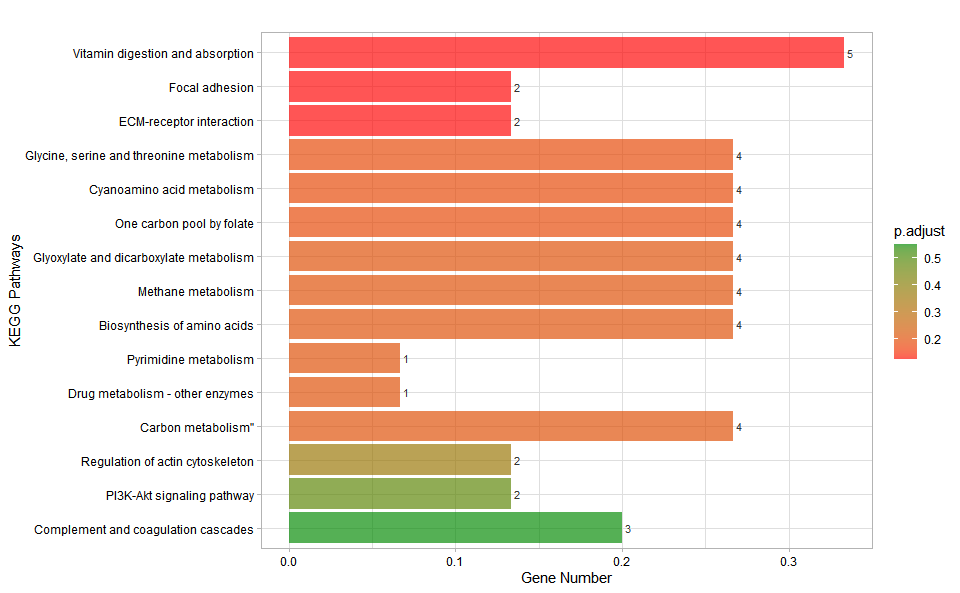
# 2. Run kegg_enrich_bar analysis function
kegg_enrich_bar(
kegg_anno,
kegg_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 1,
qvalue_cutoff = 1,
sign_by = "p.adjust",
category_num = 30,
font_size = 12,
low_color = "#ff0000aa",
high_color = "#008800aa",
ggTheme = "theme_light"
)
#> Scale for fill is already present.
#> Adding another scale for fill, which will replace the existing scale.Get help using command ?TOmicsVis::kegg_enrich_bar or reference page
https://benben-miao.github.io/TOmicsVis/reference/kegg_enrich_bar.html.
# Get help with command in R console.
# ?TOmicsVis::kegg_enrich_barKEGG enrichment analysis and dot plot based on KEGG annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(kegg_anno)
# head(kegg_anno)
data(kegg_deg_fc)
# head(kegg_deg_fc)
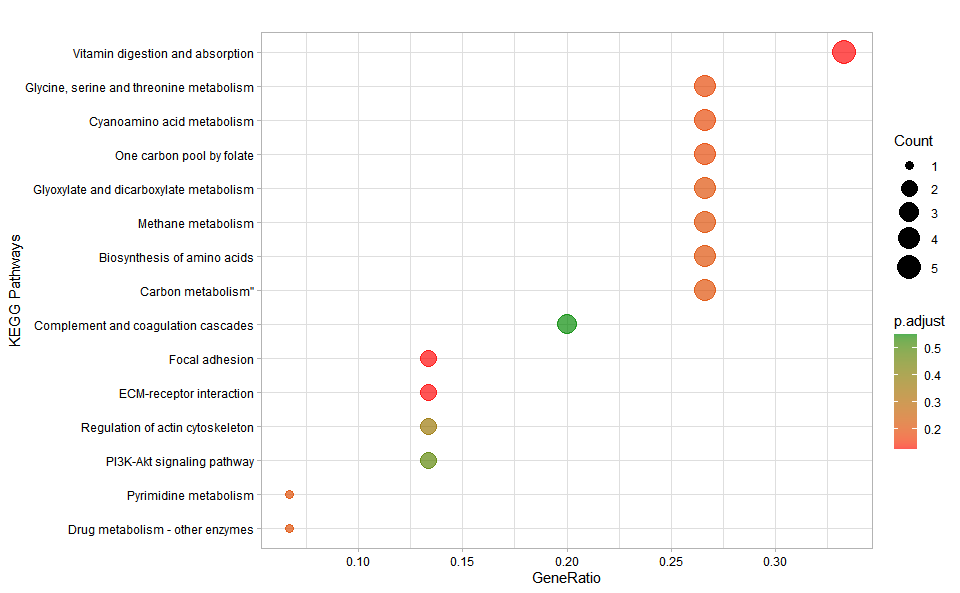
# 2. Run kegg_enrich_dot analysis function
kegg_enrich_dot(
kegg_anno,
kegg_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 1,
qvalue_cutoff = 1,
sign_by = "p.adjust",
category_num = 30,
font_size = 12,
low_color = "#ff0000aa",
high_color = "#008800aa",
ggTheme = "theme_light"
)
#> Scale for colour is already present.
#> Adding another scale for colour, which will replace the existing scale.Get help using command ?TOmicsVis::kegg_enrich_dot or reference page
https://benben-miao.github.io/TOmicsVis/reference/kegg_enrich_dot.html.
# Get help with command in R console.
# ?TOmicsVis::kegg_enrich_dotKEGG enrichment analysis and tree plot based on KEGG annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(kegg_anno)
# head(kegg_anno)
data(kegg_deg_fc)
# head(kegg_deg_fc)
# 2. Run kegg_enrich_tree analysis function
kegg_enrich_tree(
kegg_anno,
kegg_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 1,
qvalue_cutoff = 1,
sign_by = "p.adjust",
category_num = 20,
font_size = 4,
low_color = "#ff0000aa",
high_color = "#008800aa",
hclust_method = "complete",
ggTheme = "theme_light"
)Get help using command ?TOmicsVis::kegg_enrich_tree.
# Get help with command in R console.
# ?TOmicsVis::kegg_enrich_treeKEGG enrichment analysis and net plot based on KEGG annotation results (None/Exist Reference Genome).
# 1. Load example datasets
data(kegg_anno)
# head(kegg_anno)
data(kegg_deg_fc)
# head(kegg_deg_fc)
# 2. Run kegg_enrich_net analysis function
kegg_enrich_net(
kegg_anno,
kegg_deg_fc,
padjust_method = "fdr",
pvalue_cutoff = 1,
qvalue_cutoff = 1,
category_num = 20,
net_layout = "circle",
net_circular = TRUE,
low_color = "#ff0000aa",
high_color = "#008800aa"
)
#> Scale for size is already present.
#> Adding another scale for size, which will replace the existing scale.
#> Scale for colour is already present.
#> Adding another scale for colour, which will replace the existing scale.Get help using command ?TOmicsVis::kegg_enrich_net or reference page
https://benben-miao.github.io/TOmicsVis/reference/kegg_enrich_net.html.
# Get help with command in R console.
# ?TOmicsVis::kegg_enrich_netTable split used for splitting a grouped column to multiple columns.
# 1. Load table_split_data example datasets
data(table_split_data)
head(table_split_data)
#> month day variable value
#> 1 5 1 ozone 41
#> 2 5 2 ozone 36
#> 3 5 3 ozone 12
#> 4 5 4 ozone 18
#> 5 5 5 ozone NA
#> 6 5 6 ozone 28
# 2. Run table_split plot function
res <- table_split(table_split_data,
grouped_var = "variable",
miss_drop = TRUE
)
head(res)
#> month day ozone solar.r temp wind
#> 1 5 1 41 190 67 7.4
#> 2 5 2 36 118 72 8.0
#> 3 5 3 12 149 74 12.6
#> 4 5 4 18 313 62 11.5
#> 5 5 5 NA NA 56 14.3
#> 6 5 6 28 NA 66 14.9Get help using command ?TOmicsVis::table_split or reference page
https://benben-miao.github.io/TOmicsVis/reference/table_split.html.
# Get help with command in R console.
# ?TOmicsVis::table_splitTable merge used to merge multiple variables to on variable.
# 1. Load example datasets
data(table_merge_data)
head(table_merge_data)
#> Ozone Solar.R Wind Temp Month Day
#> 1 41 190 7.4 67 5 1
#> 2 36 118 8.0 72 5 2
#> 3 12 149 12.6 74 5 3
#> 4 18 313 11.5 62 5 4
#> 5 NA NA 14.3 56 5 5
#> 6 28 NA 14.9 66 5 6
# 2. Run function
res <- table_merge(
table_merge_data,
merge_vars = c("Ozone", "Solar.R", "Wind", "Temp"),
new_var = "Variable",
new_value = "Value",
na_remove = FALSE
)
head(res)
#> Month Day Variable Value
#> 1 5 1 Ozone 41
#> 2 5 2 Ozone 36
#> 3 5 3 Ozone 12
#> 4 5 4 Ozone 18
#> 5 5 5 Ozone NA
#> 6 5 6 Ozone 28Get help using command ?TOmicsVis::table_merge or reference page
https://benben-miao.github.io/TOmicsVis/reference/table_merge.html.
# Get help with command in R console.
# ?TOmicsVis::table_mergeTable filter used to filter row by column condition.
# 1. Load example datasets
data(table_filter_data)
head(table_filter_data)
#> # A tibble: 6 × 14
#> name height mass hair_color skin_color eye_color birth_year sex gender
#> <chr> <int> <dbl> <chr> <chr> <chr> <dbl> <chr> <chr>
#> 1 Luke Sky… 172 77 blond fair blue 19 male mascu…
#> 2 C-3PO 167 75 <NA> gold yellow 112 none mascu…
#> 3 R2-D2 96 32 <NA> white, bl… red 33 none mascu…
#> 4 Darth Va… 202 136 none white yellow 41.9 male mascu…
#> 5 Leia Org… 150 49 brown light brown 19 fema… femin…
#> 6 Owen Lars 178 120 brown, gr… light blue 52 male mascu…
#> # ℹ 5 more variables: homeworld <chr>, species <chr>, films <list>,
#> # vehicles <list>, starships <list>
# 2. Run function
res <- table_filter(table_filter_data,
height > 100 & eye_color == "black"
)
head(res)
#> # A tibble: 6 × 14
#> name height mass hair_color skin_color eye_color birth_year sex gender
#> <chr> <int> <dbl> <chr> <chr> <chr> <dbl> <chr> <chr>
#> 1 Greedo 173 74 <NA> green black 44 male mascu…
#> 2 Nien Nunb 160 68 none grey black NA male mascu…
#> 3 Gasgano 122 NA none white, bl… black NA male mascu…
#> 4 Kit Fisto 196 87 none green black NA male mascu…
#> 5 Plo Koon 188 80 none orange black 22 male mascu…
#> 6 Lama Su 229 88 none grey black NA male mascu…
#> # ℹ 5 more variables: homeworld <chr>, species <chr>, films <list>,
#> # vehicles <list>, starships <list>Get help using command ?TOmicsVis::table_filter or reference page
https://benben-miao.github.io/TOmicsVis/reference/table_filter.html.
# Get help with command in R console.
# ?TOmicsVis::table_filterTable cross used to cross search and merge results in two tables.
# 1. Load example datasets
data(table_cross_data1)
head(table_cross_data1)
#> geneID root_exp leave_exp
#> 1 Unigene01 16.4798 3.3122
#> 2 Unigene02 44.5027 24.1932
#> 3 Unigene03 86.9566 43.0663
data(table_cross_data2)
head(table_cross_data2)
#> geneID KO_id ko_definition
#> 1 Unigene02 K10592 E3 ubiquitin-protein ligase HUWE1
#> 2 Unigene03 K10592 NADH dehydrogenase I subunit 4
#> 3 Unigene04 K05579 dehydrogenase I subunit 7
# 2. Run function
res <- table_cross(
table_cross_data1,
table_cross_data2,
inter_var = "geneID",
left_index = TRUE,
right_index = FALSE
)
head(res)
#> geneID root_exp leave_exp KO_id ko_definition
#> 1 Unigene01 16.4798 3.3122 <NA> <NA>
#> 2 Unigene02 44.5027 24.1932 K10592 E3 ubiquitin-protein ligase HUWE1
#> 3 Unigene03 86.9566 43.0663 K10592 NADH dehydrogenase I subunit 4Get help using command ?TOmicsVis::table_cross or reference page
https://benben-miao.github.io/TOmicsVis/reference/table_cross.html.
# Get help with command in R console.
# ?TOmicsVis::table_cross