You can install the pre-2022-03-23 version with:
remotes::install_github('MSKCC-Epi-Bio/gnomeR@v1.1.0')You can install the development version of gnomeR from
GitHub with:
# install.packages("devtools")
devtools::install_github("MSKCC-Epi-Bio/gnomeR")Along with its companion package for cbioPortal data download:
devtools::install_github("karissawhiting/cbioportalr")the gnomeR package provides a consistent framework for genetic data
processing, visualization and analysis. This is primarily targeted to
IMPACT datasets but can also be applied to any genomic data provided by
cBioPortal. With {gnomeR} and {cbioportalR} you can:
- Download and gather data from CbioPortal - Pull from cBioPortal data base by study ID or sample ID.
- OncoKB annotate data - Filter genomic data for known oncogenic alterations.
- Process genomic data - Process retrieved mutation/maf, fusions, copy-number alteration, and segmentation data (when available) into an analysis-ready formats.
- Visualize processed data - Create OncoPrints, heatmaps and summary plots from processed data.
- Analyzing processed data- Analyze associations between genomic variables and clinical variables or outcomes with summary tables, advanced visualizations, and univariate models.
{gnomeR} works with any genomic data that follows cBioPortal guidelines for mutation, CNA, or fusion data file formats.
If you wish to pull the data directly from cBioPortal, see how to get set up with credentials with the {cbioportalR} package.
The below examples uses the data sets mutatations, sv, cna which
were pulled from cBioPortal and are included in the package as example
data sets. We will sample 100 samples for examples:
set.seed(123)
mut <- gnomeR::mutations
cna <- gnomeR::cna
sv <- gnomeR::sv
un <- unique(mut$sampleId)
sample_patients <- sample(un, size = 100, replace = FALSE)The main data processing function is create_gene_binary() which takes
mutation, CNA and fusion files as input, and outputs a binary matrix of
N rows (number of samples) by M genes included in the data set. We can
specify which patients are included which will force all patients in
resulting dataframe, even if they have no alterations.
gen_dat <- create_gene_binary(samples = sample_patients,
maf = mut,
fusion = sv,
cna = cna)
head(gen_dat[, 1:6])
#> ERG.fus KDM5A.fus KDM5D.fus GSK3B.fus EGFR.fus PBRM1.fus
#> P-0008869-T01-IM5 1 0 0 0 0 0
#> P-0001242-T01-IM3 0 0 0 0 0 0
#> P-0005806-T01-IM5 0 0 0 0 0 0
#> P-0007346-T01-IM5 0 0 0 0 0 0
#> P-0001861-T01-IM3 0 0 0 0 0 0
#> P-0001202-T01-IM3 0 0 0 0 0 0By default, mutations, CNA and fusions will be returned in separate columns. You can combine these at teh gene level using the following:
by_gene <- gen_dat %>%
summarize_by_gene()
head(by_gene[,1:6])
#> # A tibble: 6 x 6
#> sample_id ERG KDM5A KDM5D GSK3B EGFR
#> <chr> <dbl> <dbl> <dbl> <dbl> <dbl>
#> 1 P-0008869-T01-IM5 1 0 0 0 0
#> 2 P-0001242-T01-IM3 0 0 0 0 0
#> 3 P-0005806-T01-IM5 0 0 0 0 0
#> 4 P-0007346-T01-IM5 0 0 0 0 0
#> 5 P-0001861-T01-IM3 0 0 0 0 0
#> 6 P-0001202-T01-IM3 0 0 0 0 0You can visualize your processed and raw alteration data sets using {gnomeR}’s many data visualization functions.
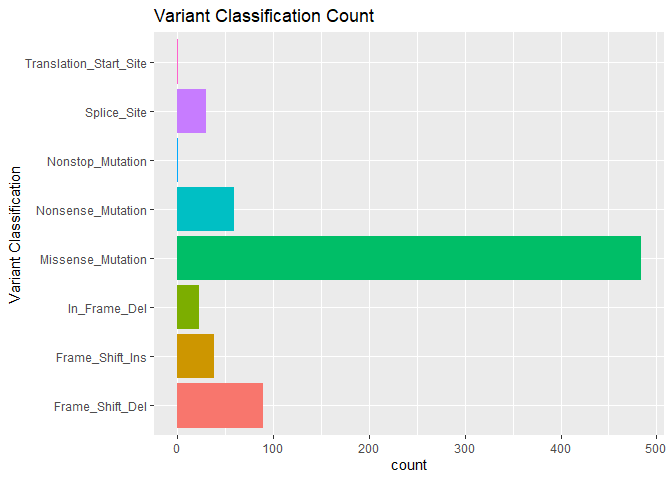
Quickly visualize mutation characteristics with ggvarclass(),
ggvartype(), ggsnvclass(), ggsamplevar(), ggtopgenes(),
gggenecor(), and ggcomut().
ggvarclass(mutation = mut)You can tabulate summarize your genomic data frame using the
tbl_genomic() function, a wrapper for gtsummary::tbl_summary().
gen_dat <- gen_dat %>%
dplyr::mutate(trt_status = sample(x = c("pre-trt", "post-trt"),
size = nrow(gen_dat), replace = TRUE)) gene_tbl_trt <- gen_dat %>%
tbl_genomic(freq_cutoff = .1, by = trt_status) %>%
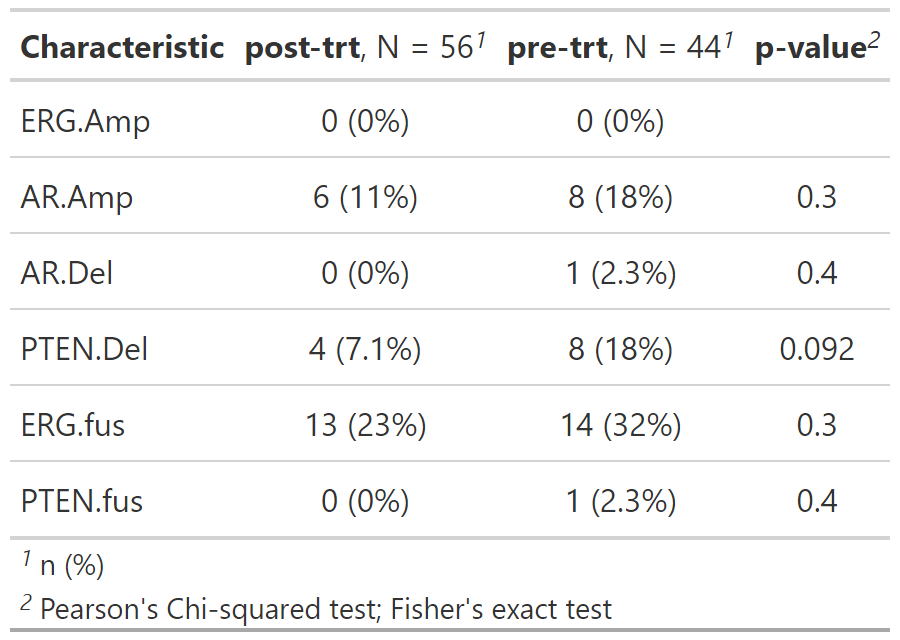
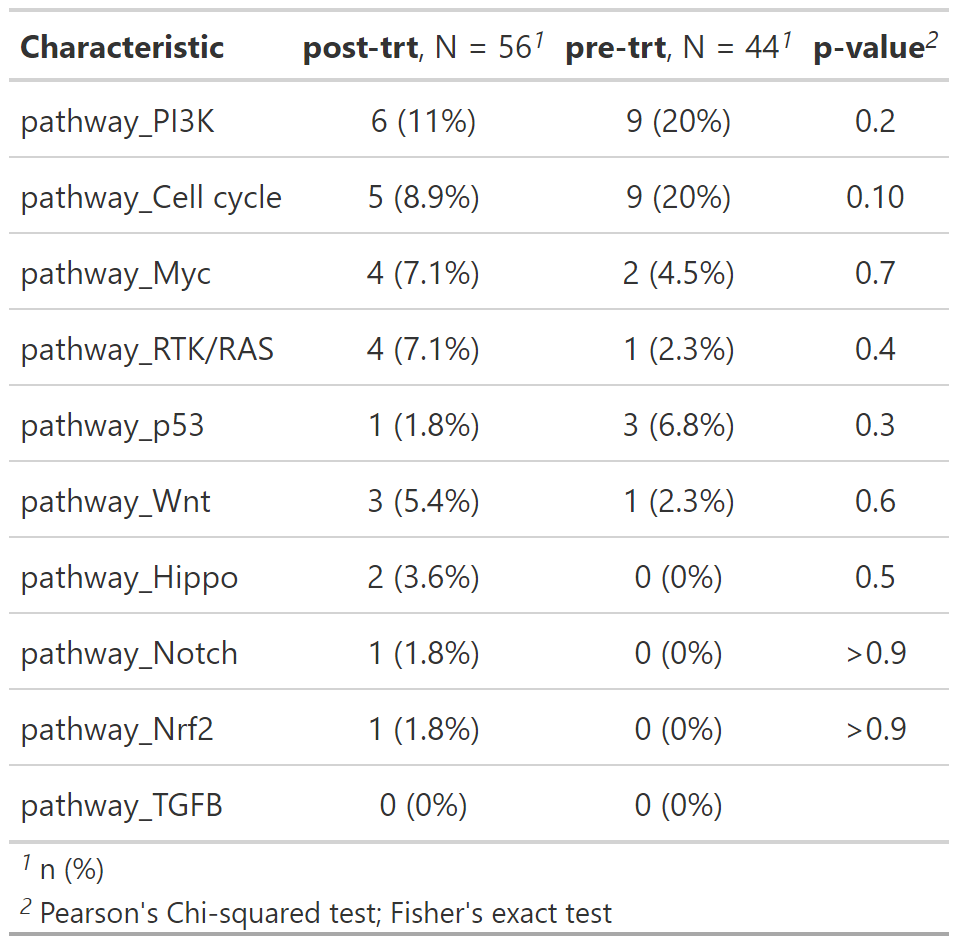
gtsummary::add_p() Additionally, you can analyze custom pathways, or a set of default gene
pathways using add_pathways():
path_by_trt <- gen_dat %>%
add_pathways() %>%
select(trt_status, contains("pathway_")) %>%
tbl_genomic(by = trt_status) %>%
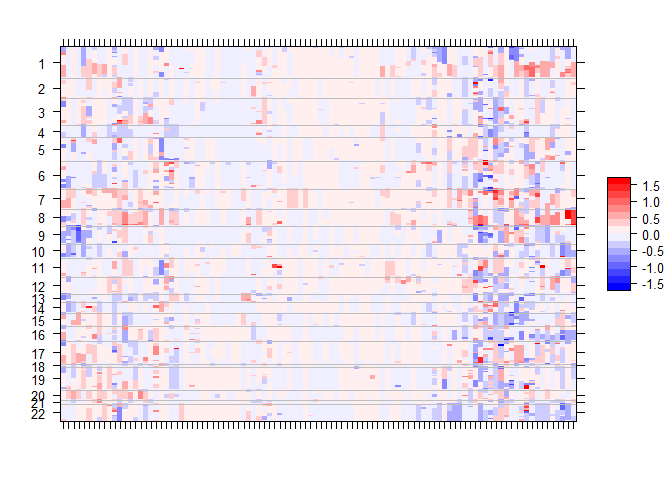
gtsummary::add_p() Along with mutation, cna and fusion data, {gnomeR} also allows analysis and visualization of FACETs data. FACETs is an allele-specific copy number tool and open-source software with a broad application to whole genome, whole-exome, as well as targeted panel sequencing platforms. It is a fully integrated stand-alone pipeline that includes sequencing BAM file post-processing, joint segmentation of total- and allele-specific read counts, and integer copy number calls corrected for tumor purity, ploidy and clonal heterogeneity, with comprehensive output.
You can visualize this data using facets_heatmap()
select_samples <- sample(unique(seg$ID), 100)
p.heat <- facets_heatmap(seg = seg, samples = select_samples, min_purity = 0)
p.heat$pPlease note that the gnomeR project is released with a Contributor Code of Conduct. By contributing to this project, you agree to abide by its terms.
Thank you to all contributors!
@akriti21, @arorarshi, @AxelitoMartin, @carokos, @ChristineZ-msk, @edrill, @hfuchs5, @jalavery, @jflynn264, @karissawhiting, @michaelcurry1123, @mljaniczek, and @slb2240