This is MemBrain v1 -- you might be actually looking for MemBrain v2, which adds more capabilities, including segmentation of membranes. If this is the case, please check out MemBrain-seg on the #TeamTomo Github.
This is the code accompanying our publication https://www.biorxiv.org/content/10.1101/2022.03.01.480844v1?rss=1 .
If you're encountering any problems when using MemBrain, feel free to reach out to us: lorenz.lamm@helmholtz-muenchen.de
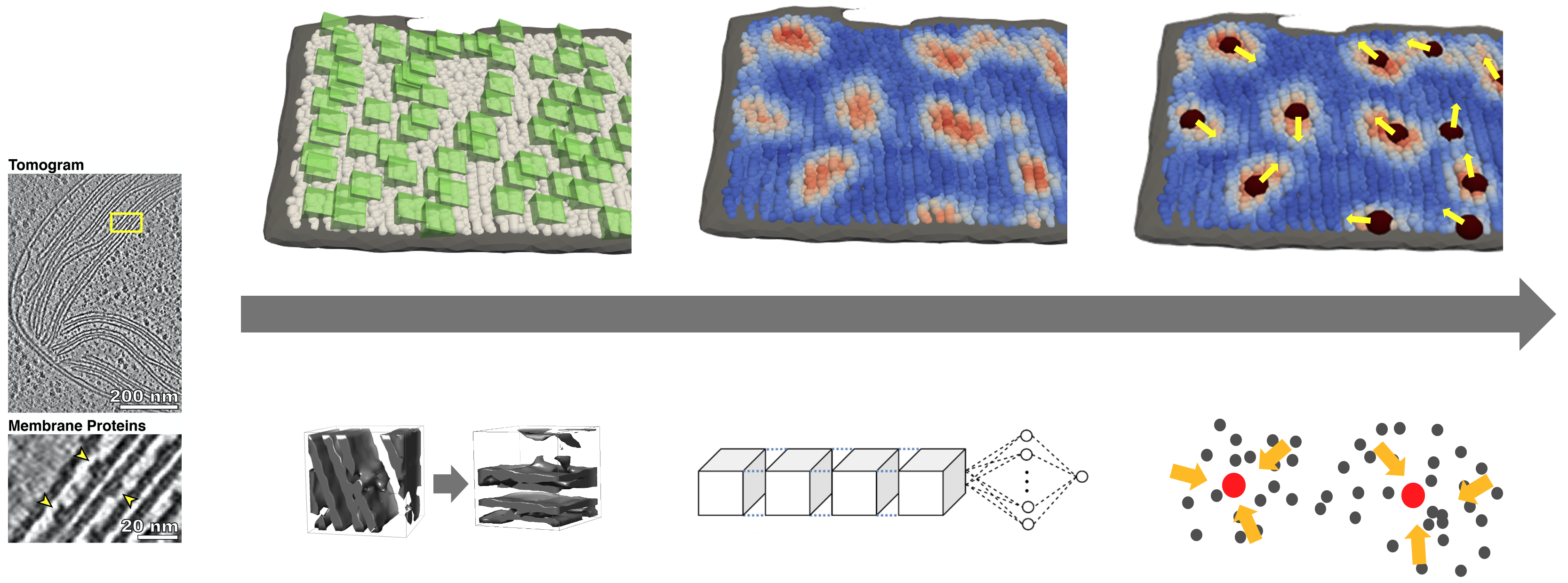
MemBrain is a pipeline for the automated detection of membrane-bound proteins in cryo-electron tomograms. It utilizes the geometry of a pre-segmented membrane to reduce the complexity of the detection task. As a result, MemBrain only requires a small amount of annotated data (even one single annotated membrane can be enough!) and can generalize well to unseen tomograms and membranes.
In this repository, we provide all code that is necessary to either
- train a MemBrain model from scratch, or
- predict particle locations on segmented membranes using a pre-trained model (see folder
./models), or - just try MemBrain out with our example dataset.
You can learn more about the workflow of our method here.
For user instructions, jump here. For instructions on how to use our example dataset, jump here. For trouble shooting, jump here.
The following instructions show the required data structure and give hints on how to properly use the scripts.
MemBrain relies on a specific data structure, where tomograms, membrane segmentations, membrane meshes, and ground truth positions should be stored: (an exemplary data structure can also be found in our example dataset)
tomograms
│
└───Tomo1
│ │ Tomo1_(...)_bin4_denoised.rec
| | Tomo1_(...)_bin4_dose-filt.rec
│ │ Remarks: "_denoised" token should be added in case you want to decide later which version to use; "bin4" token is recommended (or respective other binning)
│ │
│ └───membranes
│ │ T1S1M1.mrc
│ │ T1S18M23.mrc
│ │ T1S19M1.mrc
│ │ ...
│ │ Remarks: stack token (S1, S18, ...) and membrane token (M1, M23, ...) should be included in filenames, ending with (membrane_token + ".mrc")
| |
│ └───meshes
│ │ T1S1M1.obj
│ │ T1S18M23.obj
│ │ T1S19M1.obj
│ │ ...
│ │ Remarks: folder only necessary when training with membranorama data and using orientations; files should have names corresponding to membrane segmentations
| |
│ └───positions
│ │ T1S1M1.xml
│ │ T1S18M23.xml
│ │ T1S19M1.xml
│ │ ...
│ │ Remarks: folder only necessary for training. Files shoud be named corresponding to membrane segmentations.
|
└───Tomo23
│ Tomo23_(...)_bin4_denoised.mrc
│ Tomo23_(...)_bin4_dose-filt.mrc
For the correct Python environment, set up a virtual environment of Python 3.8 (e.g., using miniconda). When using Miniconda, the following commands can be performed to create a Python environment, after Miniconda is installed:
git clone https://github.com/CellArchLab/MemBrain.git
cd MemBrain
conda env create -f MemBrain_requirements.yml
conda activate MemBrain
In case you have a GPU available, you can run conda env create -f MemBrain_requirements_gpu.yml instead of the corresponding line above. This will create an environment that is able to make use of the GPU (for training and inference of the NN; other steps are not influenced). Activate the GPU environment using conda activate MemBrain_GPU.
Note that the virtual environment should always be activated when running Python scripts from this repository.
First, open the config file (config.py) and adjust the values according to your needs.
Hint: Setting "PICK_ON_BOTH_SIDES" to True will skip the step for manual picking of membrane sides, and process both sides of each membrane. While this setting is recommended, if graphical interfaces cannot be displayed (e.g. on a compute cluster), it is not recommended for training, as both sides use the same ground truth (which introduces probably wrong information).
Run the command
python step1_sample_points.py
This will first generate the new pipeline directory. Then, it will display the membrane segmentations one by one and lets you choose the side of the membrane to pick points on. Clicking a point with a relatively large distance to the membrane segmentation makes the side-picking more robust.
Afterwards, points and corresponding membrane normal vectors are sampled. Using the command
python step1b_inspect_picked_sides.py
you can inspect the picked sides of the membranes and verify whether they are correct.
For the extraction and preprocessing of subvolumes, run
python step2_create_subvolumes.py
In the config file, you can specify the size of the extracted subvolumes, as well as the cutoff value for the low-pass filter applied to the tomogram beforehand.
In case you don't want to use a pre-trained network, you will need to train MemBrain yourself. You can do this using
python step3a_training.py --cktp /path/to/previous/ckpt
The flag --ckpt is optional. If you want to continue training from a previously trained model, you can specify the path to that model here.
Again, you can specify training parameters in the config file, such as number of epochs, batch size, cutoff for particle distances, and particles to use for distance computation, in the config file.
Trained models and checkpoints will be stored in the folder lightning_logs.
Apply the trained model on unseen data by using
python step3b_predict_heatmaps.py /path/to/trained/model
This time, the path to the trained model is not optional, as we need to have a trained model for inference.
Executing this script will generate heatmaps for each membrane and for each particle class specified in the training parameters (config file). They are stored as .csv files and as .vtp files. The latter ones can easily be inspected, e.g. in Paraview.
Finally, we can run the script
python step4_extract_particle_centers.py
or
python step4_extract_particle_centers.py --eval True
--eval is again an optional argument. If set to True, evaluation metrics will be computed, such as the Chamfer distance between prediction and ground truth positions, as well as hit statistics based on various hit threshold distances.
Note: For --eval True to work, you will need to have corresponding .xml files for each membrane side (currently, this might not be the case in the toy example).
In the config file, you can also specify the bandwidths that should be used for clustering. (Per clustering, only one is used. Setting multiple bandwidths can help to compare them).
Outputs will be particle centers in .csv and .vtp format, stored in the folder particle_centers/raw/`
As an additional feature, we can extract estimates of particle orientations for each extracted particle position. This can be done using the command
python step5a_extract_orientations.py
For testing the functionality of MemBrain, we provide a toy dataset, containing 3 membranes from one tomogram (https://elifesciences.org/articles/53740). Instructions how to use it can be found here:
The corresponding ground truth data positions, as well as membrane meshes are provided without requiring further processing. For membrane segmentations, and the raw tomogram, please open a terminal in the MemBrain folder and run
sh prepare_toy_dataset.sh
This will automatically unzip the compressed membrane files, download the raw tomogram from EMDB (https://www.ebi.ac.uk/emdb/EMD-10780), and integrate everything into the data structure.
Ideally, this toy example should work without adjusting config file values. However, if problems with the paths arise, changing to absolute paths might help:
- PROJECT_NAME (can also stay the same)
- PROJECT_DIRECTORY (this is where all outputs of MemBrain are stored; directory should exist beforehand)
- TOMO_DIR (where your toy_data tomograms are stored, e.g.,
/path/to/MemBrain/folder/MemBrain/toy_data/tomograms) - USE_GPU (do you have GPU available? This will speed up training / inference)
The remaining instructions for this toy dataset are analogous to the common script executions, see here.
-
Loss is very high (1e2 and above): Most likely the labels have not been set correctly. Example problem for Membranorama: Membranorama stores positions based on actual pixel spacing, which it receives from a tomograms header. So if the tomogram’s header has pixel spacing 1.0 (often the case after some preprocessing with Python, e.g. CryoCARE), the Membranorama output positions will not show the exact positions w.r.t. pixel spacing. Possible solutions:
- Adjust Membranorama positions (multiply by pixel spacing)
- Set tomogram pixel spacing to 1.0 in MemBrain pipeline (will lead to further adjustments, e.g. when choosing particle radius)
-
Error
origin_pos_x = [point[0] for point in point_list] TypeError: 'NoneType' object is not subscriptableThis is an issue with the picked positions: Either, you did not click on the corresponding side of a membrane in the graphical user interface. Or (more likely), you are working on a machine that does not support the display of graphical user interfaces. Solution: Set the parameter "PICK_ON_BOTH_SIDES" in config.py to True. This will skip the manual side picking step.