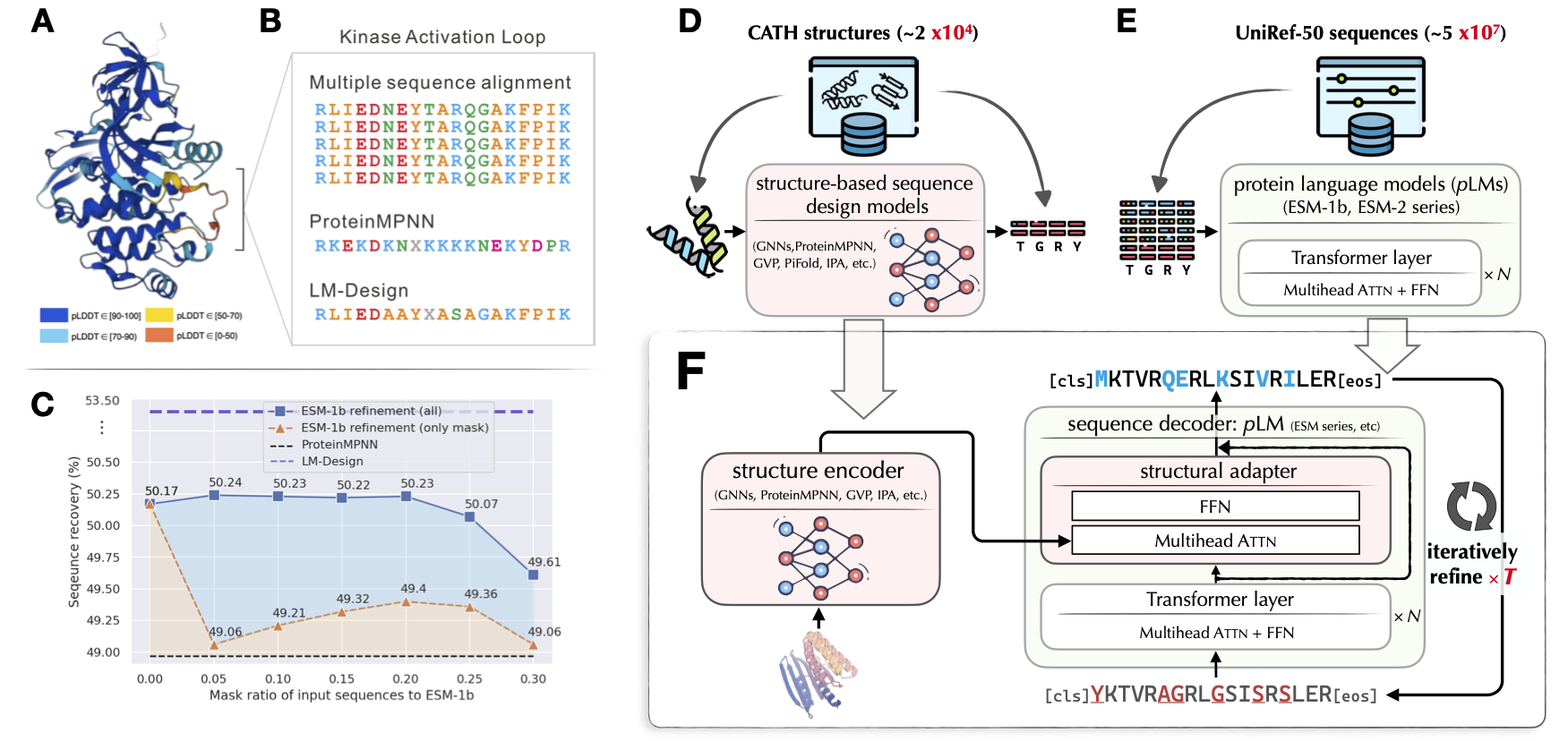
ByProt is a versatile toolkit designed for generative learning in protein research. It currently focuses primarily on structure-based sequence design (a.k.a., fixedbb), offering the following key features:
- Efficient non-autoregressive ProteinMPNN variant: ByProt provides an efficient and effective non-autoregressive variant of ProteinMPNN, a powerful tool for protein fixed-backbone sequence design.
- Official implementation of LM-Design : ByProt serves as the official implementation of LM-Design, the state-of-the-art protein sequence design model from the paper titled "Structure-informed Language Models Are Protein Designers," which was presented at ICML 2023 (oral). For more details, please refer to the paper.
We are continuously expanding ByProt's capabilities to encompass a broader range of tasks and features. Stay tuned for updates as we strive to provide an even more comprehensive toolkit for protein research.
# clone project
git clone --recursive https://url/to/this/repo/ByProt.git
cd ByProt
# create conda virtual environment
env_name=ByProt
conda create -n ${env_name} python=3.7 pip
conda activate ${env_name}
# automatically install everything else
bash install.shPretrained model weights (Zenodo)
| model | training data | checkpoint |
|---|---|---|
protein_mpnn_cmlm |
cath_4.2 | link |
lm_design_esm1b_650m |
cath_4.2 | link |
lm_design_esm2_650m |
cath_4.2 | link |
lm_design_esm2_650m |
multichain | link |
Download the preproceesd CATH datasets
- CATH 4.2 dataset provided by Generative Models for Graph-Based Protein Design (Ingraham et al, NeurIPS'19)
- CATH 4.3 dataset provided by Learning inverse folding from millions of predicted structures (Hsu et al, ICML'22)
bash scripts/download_cath.shGo check configs/datamodule/cath_4.*.yaml and set data_dir to the path of the downloaded CATH data.
Dowload PDB complex data (multichain)
This dataset curated protein (multichain) complexies from Protein Data Bank (PDB). It is provided by Robust deep learning-based protein sequence design using ProteinMPNN. See their github page for more details.
bash scripts/download_multichain.shGo check configs/datamodule/multichain.yaml and set data_dir to the path of the downloaded multichain data.
OK we now get everything ready and can start to train a model.
In the following sections, we will use CATH 4.2 dataset as an runing example. You can likewise build your models on the multichain dataset to accommodate protein complexies.
Training NAR ProteinMPNN with conditional masked language modeling (CMLM)
export CUDA_VISIBLE_DEVICES=0
# or use multi-gpu training when you want:
# export CUDA_VISIBLE_DEVICES=0,1
exp=fixedbb/protein_mpnn_cmlm
dataset=cath_4.2
name=fixedbb/${dataset}/protein_mpnn_cmlm
python ./train.py \
experiment=${exp} datamodule=${dataset} name=${name} \
logger=tensorboard trainer=ddp_fp16 Some flags for training:
| Argument | Usage |
|---|---|
experiment |
experiment config. see ByProt/configs/experiment/ folder |
datamodule |
dataset config. see ByProt/configs/datamodule folder |
name |
experiment name, deciding the directory path your experiment saving to, e.g., /root/research/projects/ByProt/run/logs/${name} |
logger |
config of which ml experiment logger to use, e.g., tensorboard. |
train.force_restart |
set to true to force retrain the experiment under ${name}. otherwise will resume training from the last checkpoint. |
Training LM-Design upon ESM-1b 650M.
Training would take approxmiately 6 hours on one A100 GPU.
exp=fixedbb/lm_design_esm1b_650m
dataset=cath_4.2
name=fixedbb/${dataset}/lm_design_esm1b_650m
./train.py \
experiment=${exp} datamodule=${dataset} name=${name} \
logger=tensorboard trainer=ddp_fp16 Building LM-Design upon ESM-2 series using exp=fixedbb/lm_design_esm2*. Please check ByProt/configs/experiment/fixedbb.
dataset=cath_4.2
# name=fixedbb/${dataset}/protein_mpnn_cmlm
name=fixedbb/${dataset}/lm_design_esm1b_650m
exp_path=/root/research/projects/ByProt/run/logs/${name}
python ./test.py \
experiment_path=${exp_path} \
data_split=test ckpt_path=best.ckpt mode=predict \
task.generator.max_iter=5Some flags for generation
| Argument | Usage |
|---|---|
experiment_path |
folder that saves experiment (.hydra, checkpoints, tensorboard, etc) |
data_split |
valid or test dataset. |
mode |
predict for generating sequence & calculating amino acid sequence recovery; test for evaluation for nll, ppl |
task.generator |
arguments for sequence generator/sampler |
- max_iter=<int> |
maximum decoding iteration (default: 5 for LM-Design, 1 for ProtMPNN-CMLM) |
- strategy=[denoise, mask_predict] |
decoding strategy. (default: denoise for LM-Design, mask_predict for ProtMPNN-CMLM) |
- temperature=<float> |
temperature for sampling. set to 0 to disable for deterministic sampling (default: 0) |
- eval_sc=<bool> |
additional evaluating scTM score using ESMFold. (default: false) |
Example 1: ProteinMPNN-CMLM
from byprot.utils.config import compose_config as Cfg
from byprot.tasks.fixedbb.designer import Designer
# 1. instantialize designer
exp_path = "/root/research/projects/ByProt/run/logs/fixedbb/cath_4.2/protein_mpnn_cmlm"
cfg = Cfg(
cuda=True,
generator=Cfg(
max_iter=1,
strategy='mask_predict',
temperature=0,
eval_sc=False,
)
)
designer = Designer(experiment_path=exp_path, cfg=cfg)
# 2. load structure from pdb file
pdb_path = "/root/research/projects/ByProt/data/3uat_variants/3uat_GK.pdb"
designer.set_structure(pdb_path)
# 3. generate sequence from the given structure
designer.generate()
# 4. calculate evaluation metircs
designer.calculate_metrics()
## prediction: SSYNPPILLLGPFAEELEEELVEENPERAGRPVPFTTEPPSPDETEGETYLYISSLEEAEELIESNRFLEAGEENNELVGISLEAIRSVARAGKLAILDTGGEAVEKLEEANIEPIVIFLVPKSVEDVRRVFPDLTEEEAEELTSEDEELLEEFKELLDAVVSGSTLEEVLEEIREVIEEASS
## recovery: 0.37158469945355194Example 2: LM-Design
from byprot.utils.config import compose_config as Cfg
from byprot.tasks.fixedbb.designer import Designer
# 1. instantialize designer
exp_path = "/root/research/projects/ByProt/run/logs/fixedbb/cath_4.2/lm_design_esm2_650m"
cfg = Cfg(
cuda=True,
generator=Cfg(
max_iter=5,
strategy='denoise',
temperature=0,
eval_sc=False,
)
)
designer = Designer(experiment_path=exp_path, cfg=cfg)
# 2. load structure from pdb file
pdb_path = "/root/research/projects/ByProt/data/3uat_variants/3uat_GK.pdb"
designer.set_structure(pdb_path)
# 3. generate sequence from the given structure
designer.generate()
# you can override generator arguments by passing generator_args, e.g.,
designer.generate(
generator_args={
'max_iter': 5,
'temperature': 0.1,
}
)
# 4. calculate evaluation metircs
designer.calculate_metrics()
## prediction: LNYTRPVIILGPFKDRMNDDLLSEMPDKFGSCVPHTTRPKREYEIDGRDYHFVSSREEMEKDIQNHEFIEAGEYNDNLYGTSIESVREVAMEGKHCILDVSGNAIQRLIKADLYPIAIFIRPRSVENVREMNKRLTEEQAKEIFERAQELEEEFMKYFTAIVEGDTFEEIYNQVKSIIEEESG
## recovery: 0.7595628415300546** Example 3: Inpainting **
For some use cases, you may want to do inpainting on some segments of interest only while the rest of the protein remains the same (e.g., designing antibody CDRs). Here is a simple example with inpaint interface:
pdb_path = "/root/research/projects/ByProt/data/pdb_samples/5izu_proc.pdb"
designer.set_structure(pdb_path)
start_ids = [1, 50]
end_ids = [10, 100]
for i in range(5):
out, ori_seg, designed_seg = designer.inpaint(
start_ids=start_ids, end_ids=end_ids,
generator_args={'temperature': 1.0}
)
print(designed_seg)
print('Original Segments:')
print(ori_seg)The output looks like:
loading backbone structure from /root/research/projects/ByProt/data/pdb_samples/5izu_proc.pdb.
[['MVKSLFRHRT'], ['DEPIEEFTPTPAFPALQRLSSVDVEGVAWRAGLRTGDFLLEVNGVNVVKVG']]
[['MTKALFRHQT'], ['ETPIEEFTPTPAFPALQHLSSVDVEGAAYRAGLRTGDFLIEVNGVNVVKVG']]
[['STESLFRHAT'], ['ETPIEEFTPTPAFPALQHLSSVDVEGVAWRAGLRTGDFLIEVNGINVVKVG']]
[['ATARMFRHLT'], ['ETPIEEFTPTPAFPALQYLSSVDVEGVAWRAGLKTGDFLIEVNGVNVVKVG']]
[['ARKAKFRRYT'], ['ETPIEEFTPTPAFPALQVLSSVDVEGVAWRAGMRTGDFLLEVNGVNVVKVG']]
[['ADARLFREYT'], ['ETPIEEFTPTPAFPALQHLSAVDVEGVAWRAGLLTGDFLIEVNGVNVVKVG']]
[['ALRALFKHST'], ['DTPIEEFTPTPAFPALQYMSSVEVEGVAWRAGLRTGDFLIEVNGVNVVKVG']]
[['MLKMLFRHYT'], ['ETPIEEFTPTPAFPALQYLSSVDIDGMAWRAGLRTGDFLIEVNGDNVVKVG']]
[['ADKALFRHHT'], ['STPIEEFTPTPAFPALQYLESVDVDGVAYRAGLCTGDFLIEVNGVNVVKVG']]
[['AAAAAFRHST'], ['KTPIEEFTPTPAFPALQYLSRVEVDGMAWRAGLRTGDFLLEVNGVNVVRVG']]
Original Segments:
[['RTKRLFRHYT'], ['ETPIEEFTPTPAFPALQYLESVDVEGVAWRAGLRTGDFLIEVNGVNVVKVG']]ByProt extends its gratitude to the following projects and individuals:
- PyTorch Lightning and lightning-hydra-template for providing a robust foundation for our development process.
ByProt draws inspiration and leverages/modifies implementations from the following repositories:
- jingraham/neurips19-graph-protein-design for the preprocessed CATH dataset and data pipeline implementation.
- facebook/esm for their ESM implementations, pretrained model weights, and data pipeline components like
Alphabet. - dauparas/ProteinMPNN for the ProteinMPNN implementation and multi-chain dataset.
- A4Bio/PiFold for their PiFold implementation.
- jasonkyuyim/se3_diffusion for their self-consistency structural evaluation implementation.
We express our sincere appreciation to the authors of these repositories for their invaluable contributions to the development of ByProt.
@inproceedings{zheng2023lm_design,
title={Structure-informed Language Models Are Protein Designers},
author={Zheng, Zaixiang and Deng, Yifan and Xue, Dongyu and Zhou, Yi and YE, Fei and Gu, Quanquan},
booktitle={International Conference on Machine Learning},
year={2023}
}