FlashFry is a fast and flexible command-line tool for characterizing large numbers of potential CRISPR target sequences. If you're looking to characterize a smaller region or would like a nice web interface we recommend the crispor.org website.
Sections:
Quickstart
From the UNIX or Mac command line, download the latest release version of the FlashFry jar file:
wget https://github.com/aaronmck/FlashFry/releases/download/1.7.2/FlashFry-assembly-1.7.2.jardownload and then un-gzip the sample data for human chromosome 22:
wget https://raw.githubusercontent.com/aaronmck/FlashFry/master/test_data/quickstart_data.tar.gz
tar xf quickstart_data.tar.gzthen run the database creation step (this should take a few minutes, it takes ~75 seconds on my laptop):
mkdir tmp
java -Xmx4g -jar FlashFry-assembly-1.7.2.jar \
--analysis index \
--tmpLocation ./tmp \
--database chr22_cas9ngg_database \
--reference chr22.fa.gz \
--enzyme spcas9nggNow we discover candidate targets and their potential off-target in the test data (takes a few seconds). Here we're using the EMX1 target with some random sequence flanking the target site:
java -Xmx4g -jar FlashFry-assembly-1.7.2.jar \
--analysis discover \
--database chr22_cas9ngg_database \
--fasta EMX1_GAGTCCGAGCAGAAGAAGAAGGG.fasta \
--output EMX1.outputfinally we score the discovered sites (a few seconds):
java -Xmx4g -jar FlashFry-assembly-1.7.2.jar \
--analysis score \
--input EMX1.output \
--output EMX1.output.scored \
--scoringMetrics doench2014ontarget,doench2016cfd,dangerous,hsu2013 \
--database chr22_cas9ngg_databaseCommand line options
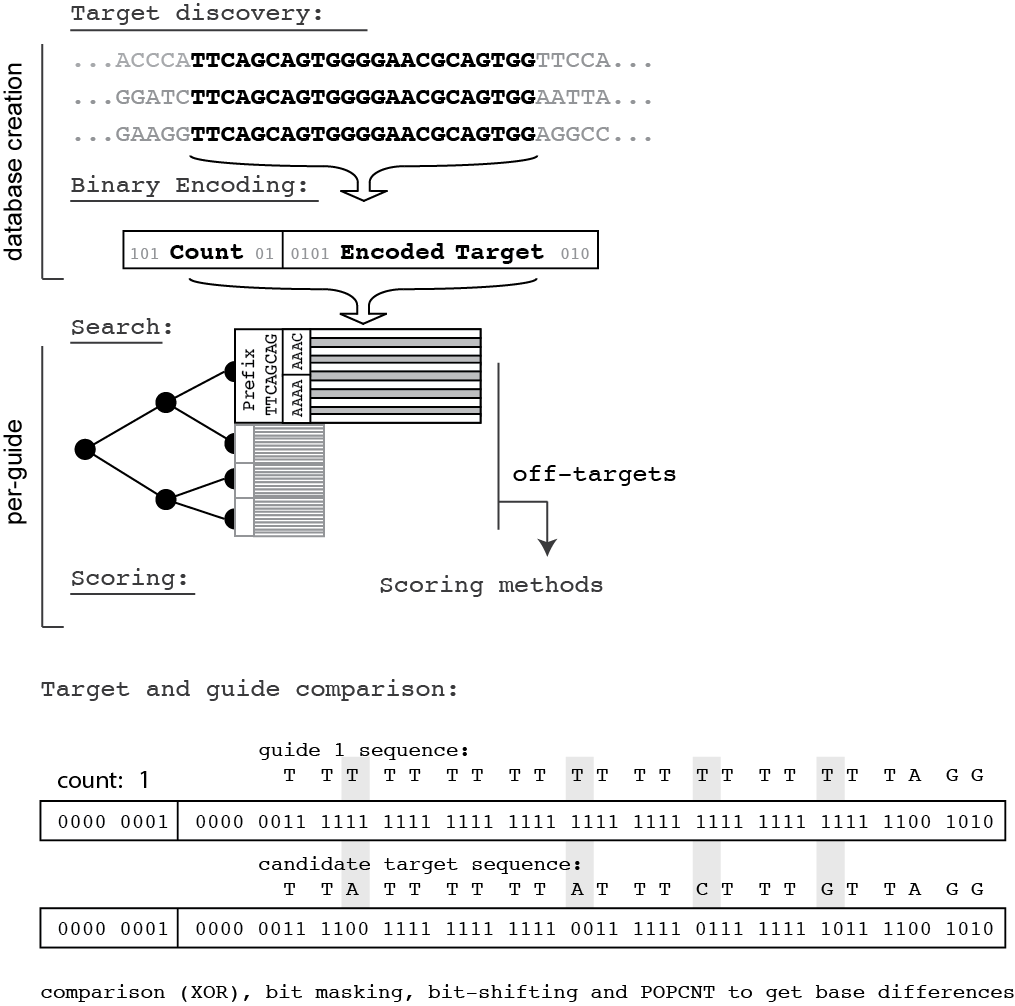
Modules are chosen using the --analysis option. Three analysis modules are currently supported, index, discover, and score. The first, index creates an off-target database from the reference genome of interest. discover takes a candidate region of interest as a fasta file and discovers CRISPR guide candidates. Lastly score produces on and off-target activity scores using schemes developed by the genomics community. Each module's options are listed below:
--analysis index
reference (required)- the input fasta reference file you'd like to discover off-target sequences indatabase (required)- the output database file to generateenzyme (optional, default to spcas9)- which enzyme to consider when making discovering sites in the reference. Options include:cpf1- 24 base targets with a 5' TTTN sequencespcas9- 23 base targets with a 3' NRG (NAG/NGG) sequencespcas9ngg- 23 base targets with a 3' NGG sequencespcas9nag- 23 base targets with a 3' NAG sequence
binSize (optional, default to 7)- what bin size should we use when indexing the fasta file. Originally this had a bigger effect on search speed, but now individual bins can have their own lookup tables, reducing the importance of this parameter.
--analysis discover
fasta (required)- the input fasta file you'd like to discover target sequences indatabase (required)- the database of off-target sequences for the genome of interestoutput (required)- the output filepositionOutput (optional, default to false)- should we output positional information along with the off-target sequences? This can make really, really large filesforceLinear (optional, default to false)- this forces FlashFry to perform a linear traversal instead of a precomputed bin traversal of the database. The only reason to use this is the case where you have a large number of guides (>10K or so), in which case it saves the time it takes FlashFry to realize it needs to do a linear traversal anyway.maxMismatch (optional, default to 4)- the mismatch threshold to consider for off-target discoveryflankingSequence (optional, default to 10)- how much sequence context to preserve up and downstream of the target. This sequence context is used by on-target metrics.maximumOffTargets (optional, default to 2000)- the number of off-targets to store before marking a candidate with the "OVERFLOW" tag. Lower values here speed up search and keep memory requirements low, higher values do the opposite. I'd recommend keeping this at the default for initial searches, and only raising it if you don't get a rich enough candidate list or you're doing this for methods development.
--analysis score
input (required)- the input file produced by thediscovermoduleoutput (required)- the scored output filedatabase (required)- the database of off-target sequences for the genome of interestmaxMismatch (required)- the maximum number of mismatches in off-targets to consider. This is a way to filter down the mismatch list considered in thediscovermodule output (say you ran that with 5 mismatches considered indiscover, but now you only want to consider 3)scoringMetrics (required)- which scoring metrics to apply. See below for the supported scoring options.
Scoring methods
The following scoring options can be supplied to the --scoringMetrics command line parameter. I'd recommend reading Read Haeussler et al. for details about scoring schemes, and which are most appropriate given your experimental design Link. Some of these scores have command line options of their own, documented below. A reminder that FlashFry currently outputs guides that OVERFLOW. You should exclude these from your analysis, are are only included for completeness (their scores are not valid):
-
hsu2013(ranking score) - Also known as the crispr.mit.edu score. From the paper "DNA targeting specificity of RNA-guided Cas9 nucleases" Hsu et. al. Nature Biotechnology, 2013 Pubmed link .This score is valid over the NGG and NAG Cas9 targets. Although the original website has some issues, this is probably the most widely used off-target specificity score. Scores range from 0 to 100, higher scores are better. Scores will vary for a guide sequence with the allowed number of mismatches. -
doench2014ontarget(ranking score) - On-target activity score from "Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation". Doench et. al. Nature Biotechnology, 2014 Pubmed link. From 0 to 1, higher scores mean more active guide sequence. -
doench2016cfd(ranking score) - The Doench 2016 cutting frequency determination score, a measure of how well an off-target candidate in the genome will be cut by your guide. The scores here range from 1 (highly active cutting at the off-target) to 0 (not active). This is calculated for each off-target, and we take the highest score (see FAQ for more details) Pubmed -
moreno2015(ranking score) - Moreno-Mateos and Vejnar's CRISPRscan on-target scoring scheme: Pubmed. Scores range from 1 (highly active at their target) to 0 (not an active cutting guide). Negitive values are possible. Read Haeussler et al. for caveats about this score Link -
rank(meta score) - This score takes the rank-ordering of any scoring metric included above, finds the median rank, and then ranks all of the guides by their median rank. Scores that are undefined (such as on-target scores that aren't given enough sequence context to score) are tied at the worst rank before calculating the median rank. The top 1000 guides are then ranked using the Schulze voting method. This is intended to help users pick the best aggregate targets across multiple scoring schemes. This is still a bit experimental. -
bedannotator- annotate the scored output file with associated annotations from a BED file. Additional command line options:inputAnnotationBed: the bed file to pull annotation information from.transformPositions: The bedannotator module will attempt to assign annotations by transforming the candidates within the target regions from the bed into the cordinate space specified. Say if you pulled your region from the 1Mb superenhancer region in front of the human MYC gene, which you called>MYC_Regionin the fasta file. You would then include a BED file where you had a line likechr8 127000000 128000000 MYC_Region(seperated by tabs) here, and the bed annotator would transform each candidate guide into this coordinate space using the start and stop of the line as offsets into this space.
-
dangerous- annotate sequences that would be difficult to work with. Currently this includes:IN_GENOME=X: The number of times a perfect match target for this guide sequence is seen within the genome of interest.GC_X: flagging sequences that have a high (>75%) or low (<25%) GC contentPolyT: guide sequences (subsetted from the target sequences) sequences that have four or more thymine (T) bases in a row. Could potentially terminate polIII transcription early (not an issue with other transcription approaches)
-
minot- a convenience score: what's the minimum distance to the target within the off-target set? encodes both the distance and the number of off-targets at that distance -
reciprocalofftargets- mark guides within the target region that are a good off-target to one-another. This can lead to large deletion drop-out, which can confound results
General documentation
FlashFry requires the Java virtual machine (JVM) to run. This is on almost every system imaginable these days, so it's probably already on your machine. We've tested it with both Oracle's Java as well as using the open JVM. Other requirements include:
- Your reference genome as a fasta file
- The region you'd like to score, as a fasta file
- A computer, with the command line terminal open
- Java 1.8
Once you have the requirements setup, there are three main steps to running FlashFry.
- First, you build a database using the specified CRISPR motif against the target database using the
--analysis indexoption. This is only done once, as the database is reuseable. You have to choose the enzyme type to use while indexing. As of writing this includes the Cas9s with 23 bp targets: SpCas9 (NAG or NGG), SpCas9NGG (NGG), SpCas9NAG (NAG), and Cpf1 (TTTN) with 24 basepair targets. These are adjustable in the code, or you can create your own. In writing the database temporary files are put in the --tempLocation location. This will take up a bit more space than the final database (maybe 10-20% depending on how duplicated genome targets are). Runtimes on a pretty slow drive look like (formated hours:minutes:seconds):
| Genome / version | Cas9 (NGG) | Cas9 (NGG/NAG) | CPF1 (TTTN) |
|---|---|---|---|
| Caenorhabditis elegans - 235 | 0:3:21 | 0:6:03 | 0:5:35 |
| Human - hg38 | 3:19:29 | 5:24:55 | 2:50:59 |
| Mouse - mm10 | 2:36:53 | 4:36:03 | 2:11:35 |
| Drophellia melanegaster - BDGP6 | 0:6:33 | 0:10:48 | 0:5:44 |
-
The next step is to find candidate targets within the fasta sequence of interest. The
--analysis discoveroptions handles this. The candidates found in the fasta are then run against the off-targets database, and an annotated output file is produced. This output file is a tab-delimited text file. -
Lastly, you can score this annotated output file. This is handled by the
--analysis scoremodule. We've implemented a fair number of scoring metrics. For guidance on which are appropriate in which situation, please see the wonderful paper by Maximilian Haeussler which analyzed all of these methods in aggregate:
hsu2013- The Hsu et. al. method, also known as crispr.mit.edu score: Pubmeddoench2014ontarget- Doench 2014 on-target efficiency score Pubmeddoench2016cfd- The Doench 2016 cutting frequency determination score Pubmedmoreno2015- Moreno-Mateos and Vejnar's CRISPRscan on-target method Pubmed
We've also implemented a some additional metrics that are useful in CRISPR library creation:
bedannotator- annotation your output targets with information from an associated BED file. It can find the 0 mismatch targets in the genome database and use those to infer the genomic locationdangerous- annotate targets that have dangerous sequence features, such as high or extremely low GC, polIII transcriptional terminators, or low entropy.minot- add a column that indicates the minimum mismatches to any off-target hit.
Binary file format
The tool uses a custom binary format that compresses the genome hits for a target sequences into binary values. Values are stored as using Java's default big-endian format. The database itself contains all the packed long values in a compressed file, using htslib's block-compressed file readers and writers. The header file sits alongside the database and provides a lookup table for the target database.
The block format in the database is:
- block type: currently 0 for linear, 1 for sub-indexed (long)
- if indexed, for the set index size, a series of long values that describe the size and location of sub-indexes
- After any header, a series of targets, with X position encodings, set by the count number in the target encoding
The header format:
-
64 bit magic value (long)
-
64 bit version number (long)
-
64 bit internal enzyme number (long, see StandardScanParameters.scala for the enumerated enzymes)
-
64 bit number of bins (long)
-
for the number of bins in the header:
- 64 bit offset of this block in the file (long)
- 64 bit size of this block in the file (in uncompressed bytes, long)
- 64 bit number of targets contained within the bin (int)
-
for each contig in the input reference file:
- the contig name, followed by a '=' and it's index position in the reference
For lookup we perform something like the following:
FAQ
Why seperate the off-target discovery and scoring modules of FlashFry?
Off-target discovery can have high computational costs for large putitive target sets (say 10,000 to 100,000s of candidate guides). To avoid having to do this step every time you'd like to switch scoring metrics, we thought it was best to split the two stages up. You can also discover sites for the largest mismatch theshold you'd like to use, and then filter this down in scoring steps.
Why the does the output file look the way it does?
We orignially wanted the output to work with common analysis tools such as BEDTools. This meant a format that encoded specific details into BED-file columns, as well as leaving off a traditional header line in favor of listing column details in the header section. In the end this had limited utility, especially when we added the capability to annotate the output with BED files directly.
How much memory should I give FlashFry?
The memory requirements of FlashFly are determined by the number guides you're looking at and the number of off-targets you allow per guide candidate. The first factor is controlled by the size of the region you're looking at, and the second is controlled by the --maximumOffTargets parameter in the discovery phase. Generally with < 100K guides and --maximumOffTargets set to 2000 you'll be able to run with 4g of memory or less (such a memory limit is set in the JVM with the -Xmx4g command line parameter, right after java). You will need to increase this number with higher guide counts, a higher mismatch thresholds, or if you want to retain more off-targets.
Why are some scores NA?
If the scoring metric is unable to produce a score for the specified guide it will output NA. This commonly happens when there isn't enough sequence context on either side of a guide for the on-target scoring, which can occur if the guide sits near the beginning or end of the input fasta file.
Should I use targets marked with OVERFLOW?
No. We stop accumulating statistics on those targets after we've found too many candidate off-target sites. The remaining numbers are not reliable; these targets are kept in the output as a reference, a look-up for sites you expect to find but are poor candidates.
Should I use a masked or unmasked genome to build my database?
Masking a genome obscures repetitive bases by either converting them to Ns (hard-masked) or making them lowercase (soft-masked). We recommend using a unmasked or soft-masked genome. You generally want to consider repetitive content when designing guides (you'd like to know about any off-targets within the genome), and this is not possible with hard-masked genomes.
How do we score the CFD from Doench 2016?
The CFD scores describes how likely a guide is to cut a specific off-target, with 1 being an exact match, and 0 being no activity. It's a little unclear of the best way to combine this over a set of off-targets. For instance if a guide edits one off-target site with a score or 0.8 and another off-target with a score of 0.2, what score do we use for the guide? We currently list the highest score -- the score from the off-target that's the most likely to be edited. It would be possible to use an aggregation score similiar to the crispr.mit.edu, where all off-targets upweight the overall score.

